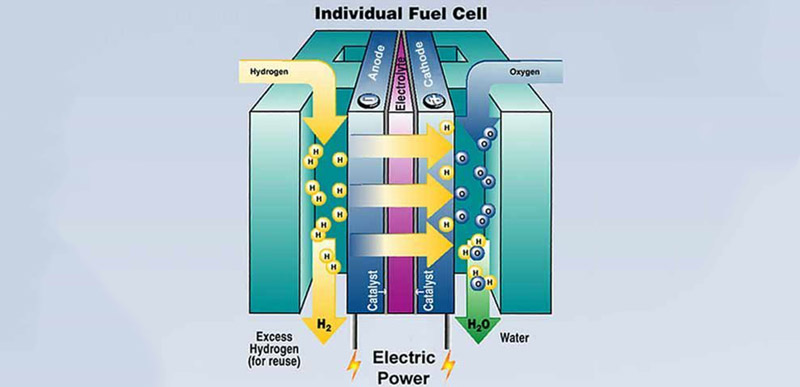
Modern hydrogen fuel cells are incredible pieces of engineering. While a simplistic diagram of a fuel cell is just a stream of hydrogen, an anode, cathode, and a bit of oxygen, this does’t convey the complexity of the most important part of the fuel cell – the proton exchange membrane.
The proton exchange membrane is the part of a fuel cell that takes in hydrogen, spits out electrons, and produces water. They can be made from platinum to expensive DuPont products, and if [Charlie]’s hypothesis is correct, stuff you can pull out of a junkyard.
The goal of [Charlie]’s Prize entry is to create a small, proof of concept fuel cell that’s safe, low cost, and very easy to build. Right now he’s focused on finding a cheap, readily available proton exchange membrane to make this build accessible to everyone.
A hydrogen fuel cell will of course have pressurized hydrogen in it, and [Charlie] is taking some steps to mitigate the risks of having his limbs blown off. His first real project update is about the safety considerations of working with hydrogen. He’ll be using a simple hydrogen gas sensor to measure for leaks and sound an alarm.


















Yeah, well, good luck.
It’s like noting that getting to low earth orbit is extremely expensive, so the idea is to find a cheap, safe, low cost and easy to build Soyuz rocket… but let’s start with an arduino and a g-force sensor.
Yo made my day :D
I’ve always liked the idea of the hydrogen fuel cell, it creates energy and its only byproduct is water. Unfortunately hydrogen is a very light gas that doesn’t hang around in our atmosphere very long. Last time I checked it took more energy to make hydrogen, than the amount of energy it could produce. I hope this is no longer the case. PS please post a link to pics of Charlie’s prototype.
There isn’t any prototype.
It’s just a “If I had a cheap PEM available”.
Most real fuel cell installations run by reforming methane into hydrogen. Seems to be the cheapest method, but has it’s inefficiencies and of course still relies on fossil fuels. http://en.wikipedia.org/wiki/Methane_reformer
Since when is methane a fossil fuel? While it certainly CAN be pulled from the ground, it can also be pulled from the waste of farm animals and even human beings. It is an infinitely renewable resource that is barely been tapped.
Waste derived methane contains a lot of CO2 which will produce carbon monoxide when reformed, and any trace amount of CO with the hydrogen will poison the fuel cell catalysts.
Separating the two is a bit costly.
Of course it still takes more energy to create the hydrogen!
Your first mistake is thinking that a fuel cell “creates” energy. The amount of energy and matter in the universe is constant. You can convert between the two (nuclear power) but that’s it. You cannot create either. All a fuel cell is doing is converting between stored chemical energy and electricity. Also.. as with any conversion process.. there is going to be entropy (some energy is just lost to heat).
Otherwise a fuel cell could be used to provide the energy to create it’s own hydrogen. That would be a perpetual motion machine which would violate the first law of thermodynamics. Even just breaking even would require 100% efficency in both conversions plus transmission of power. I believe that would violate the second law of thermodynamics.
This doesn’t make a fuel cell necessarily useless. Hydrogen might be easier to store and transport than other forms of energy such as electricity. It is less poluting than fuels such as gasoline or diesel (although this depends on how the hydrogen is produced).
By moving the pollution producing part of the chain (creating the hydrogen) to large plants rather than say.. burning gasoline in every driver’s individual car it might be possible to better control the process to produce less emissions. Ever see a big cloud coming out of someone’s old, poorly maintained car? Regulators can more easily force a few big plants to do tune-ups than millions of individuals.
Some have even proposed using sunlight to split the hydrogen from the water. Then again, you can produce electricity directly that way too. Anybody care to comment on which is more efficient?
The Laws of Thermodynamics
0: There is a game.
1: You can’t win.
2: You can’t even tie. Unless it’s very, very ,very cold. It never gets cold.
3: You must play
And here I am trying to simulate neurotransmitters in a neural net. I raise my beer mug and hope him luck on his project.
You need both platinum and the expensive PEM for a fuel cell to work. The specific DuPont product typically used is Nafion IIRC. I played around with fuel cells when I was at school and was lucky enough to score an A4 size sheet of the stuff from a local research firm.
In the end the PEM is just a solid electrolyte. There are liquid electrolyte style fuel cells out there. They are not as popular because they are not as robust and scale terribly. Go read what Wikipedia has to say on this.
As for the catalyst, if I remember correctly you can get semi decent results from Nickel if Platinum is hard to get. I was lucky enough to get my local university to plate a piece of carbon fiber mat with platinum that I used as my catalyst.
Fuel cells are real fun to play with and it was an excellent learning opportunity for me to explore something interesting. This was in the 00’s mind you, so fuel cells were pretty much still a buzzword technology.
In the end I came to the conclusion that although fuel cells can be made at home, it’s not viable for the home gamer and the processes and materials involved in making a useful one is better left to the folks with lots of money and proper manufacturing capability.
If he’s going the route of recycling old mufflers, he might be on to something. If he somehow discovers a better way to do so, it would at least add greater value to the muffler than just extracting the platinum out of it.
But junkyards aren’t allowed to sell catalytic converters 1.) Because selling used emissions equipment is a pretty big offense and 2.) The metal is too valuable and is immediately sold.
1 – wrong and 2- while true is still very wrong.
I have bought used Catalytic converters from a junkyard, they are worth more as a used working part than the metal inside is. It is not illegal to sell used emissions parts if you have the proof that it came off a car your company bought and is parting out. if you are doing it out of the trunk of your car, that is another story.
Brand new high flow catalytic converters are dirt cheap. http://www.summitracing.com/parts/sle-m310193500 $95.00 for a brand new one that is a “performance” model that has more of the platnium in it to react faster and pass any tests. the old used OEM one in a junkyard, I have bought for $45.00 when I could not afford car parts was readily available and not hard to buy. Worked great, I passed the smog test with the used part and went on my way. The metal inside is worth about $10.00 per OEM style cat, so they prefer to try and sell them for $35-$45 instead of the $10 and spending $6.00 paying a minimum wage kid to rip them open to get it out.
This won’t fly in Michigan. Junkyards won’t sell used catalytic converters here. They just send them off to be recycled. The junkyards will claim that it is against the law for them to sell a used converter, but it is only against Michigan law to install a used converter from one car onto a different car. They will also claim that it is illegal to recycle the contents of a converter for another use, but again, that’s bull.
I agree, the palladium or platinum / palladium content is worth far less than you will pay for them. Most of the weight of the honeycomb inside is alumina matrix.
Where do you live?
I don’t know this for a fact but that sounds like the kind of law that would vary from state to state here in the US. Outside the US.. I have no idea. Don’t forget this is an international forum.
We do not all live under the same set of rules!
“Wait… did you already cut the copper tubing from the back of the fridge”
Well, good luck! I know about a few universities, that tries to use a cheap material instead of the expensive one for about 10 years and a lot of money.
Hydrogen is not explosive, the hindenburg did not explode, the risk of explosion is only if he compresses the gas with Oxygen and then tried to ignite it INSIDE of a tank or vessel that does not let the pressures vent.
Unless he did everything very wrong and dumb the risk of explosion is incredibly small.
Hydrogen has an extremely wide explosive limit (4-75%) that has nothing to do with the pressure exerted on it. Wider than methane or propane. Risk of explosion depends on the quantities around. It’s not hard to prevent, but accidents are easier than you’re implying.
Also, don’t forget that hydrogen/oxygen mix is far more prone to actual detonation then simple hydrocarbons ;-)
Both the article and [Charlie] are conflating PEMs and membrane electrodes. There are many materials out there that act as a PEM.. The magic happens not in the PEM but in the catalyst that splits hydrogen to individual protons. Common PTFE plumbers tape (available in wide format) makes a fine PEM. A little bit of QC on your part to make sure there aren’t any pinholes or thin spots. A PEM is of no use if you can’t catalyze the reaction.
We’re at a bit of an impass currently. We lack the technology to generate hydrogen cleanly but until there’s a product that uses it there’s no market force to push hydrogen generation beyond hydrocarbon cracking or water electrolysis. Both of which just push the emissions to someone else. Municipal waste water could be used in a methane digester or nuclear power for electrolysis but in the end even with a steady supply of hydrogen it’s not a very dense energy source compared to hydrocarbons (someone mentioned isobutanol on another post which can be used in gasoline equipment).
hydrogen is a clean way to store surplus energy generated from solar, wind etc I’d think. even if your method isnt efficient its better than dumping the power down the terlit.
If you are connected to the grid, then there is always a customer out there willing to buy your surplus power. If not, batteries.
Batteries are expensive and wear out, a nickel-rare earth metal storage tank will last a very long time and an electrolyzer is cheap…
If electric cars will ever become dominant (a very big if :P), fuel cells will probably be the only usable way for a long-range car…
“The hope in successfully achieving the project goal, would be that electrical power could be delivered to people in remote or impoverished locations, that would otherwise not have access to electricity.”
Storing and transporting hydrogen is very difficult. Compressing it to a liquid, so can be stored in a reasonable amount of space, uses a significant portion of the maximum energy you can recover from it. I forget the exact amount, perhaps 30%? Combined with the other losses involved in generating the hydrogen, then converting it back to electricity, hydrogen is an incredibly wasteful means of storing energy.
There are some other issues with hydrogen as well, including materials compatibility. Look up “hydrogen embrittlement”. It’s something even big chemical plants have issues with.
So even if this project somehow results in a significant advance in DIY fuel cells, it will not result in the stated outcome. I don’t mean to discourage [Charlie] from experimenting, but keep it realistic.