Breaking a stud or a bolt is a pretty common shop catastrophe, but one for which a fair number of solutions exist. Drill it out, shoot in an extractor, or if you’re lucky, clamp on some Vise-Grips and hope for the best. But when a drill bit breaks off flush in a hole, there aren’t a lot of options, especially for a small bit. If the stars align, though, you may follow this video guide to dissolve the drill bit and save the part.
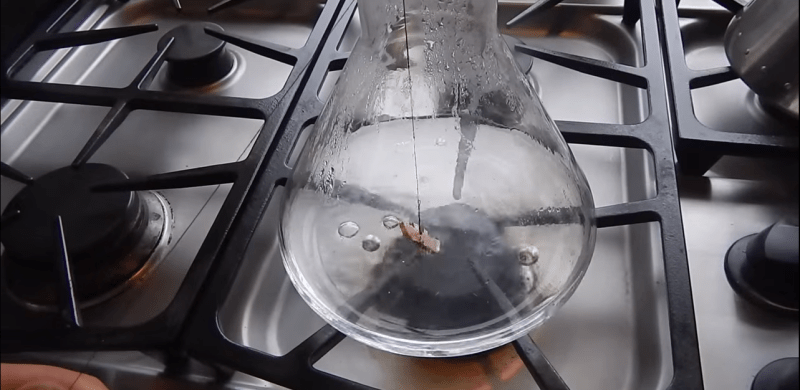
Looks like [Adam Prince] lucked out with his broken bit, which he was using to drill the hole for a pin in a small custom brass hinge. It turns out that a hot solution of alum (ammonium aluminum sulfate), which is available in the spice rack of your local supermarket, will dissolve the steel drill bit without reacting with the brass. Aluminum is said to be resistant to the alum as well, but if your busted bit is buried in steel, you’re out of luck with this shop tip.
We’re a bit disappointed that [Adam]’s video ends somewhat abruptly and before showing us the end result. But a little Googling around reveals that this chemical technique is fairly well-known among a group that would frequently break bits in brass – clockmakers. It remains to be seen how well it would work for larger drill bits, but the clocksmiths seem to have had success with their tiny drills and broaches.
As for the non-dissolved remains of the broken bit, why not try your hand at knife making?













Well, I broke a bolt in my lawn mower on saturday…
I wonder if the pulley housing it is stuck in is aluminium.
I find that screw/bolt extractors are real good at breaking off and filling the hole in the broken bolt with a piece of hardened steel. (sigh!)
Oh, I don’t think I have a pyrex beaker large enough to hold the pulley assembly B^)
Polypropylene, no need for fancy glass that ends up broken anyway.
Before you ask, it doesn’t need to be heated just warmed and it will take a few hours to a day.
You can use an aluminum pot or an aluminum baking pan. Just make sure it is not steel.
From what I saw when I researched this, a bit or bolt can be removed from a large (i.e., non-submersible) non-ferrous assembly by creating a dam with clay or Silly Putty. Basically you make a little funnel that lets you pool alum solution over the broken part. I’ve never tried it and hope I never need to, but it’s certainly one to file away just in case.
Magnet? I mean to test if it’s aluminum. Doesn’t work on some stainless, but they’re probably not using stainless inside a lawnmower…
Yeah, I’ll check it with a magnet, I’m just not sure at this moment, or when I posted the comment, what the housing is made of. Thanks.
Magnet only works for Cobalt, Iron and Nickel fyi.
Before someone gets confused, Steel is usually included in this since its made from primarily Iron.
Yup never ever use an easy out,they are junk .Old timer showed me how to remove broken studs using an Alan or Hex key ,drill bolt a fraction under across the points of the hex hammer alan key in and wind out the offending bolt. if the key breaks it will break on the bend not flush like an easy out
It should work just fine for larger bits. Even better if the break is in a portion of the bit w/ the flutes. You only need to dissolve a few thou of steel around the circumference to be able to remove it with some pliers, no need to dissolve the whole bit. If the break is along the shank, mechanical rather than chemical means, is probably faster. Using a carbide or diamond bit to drill and then tap a hole might allow you to insert a bolt/threaded rod to pull it out.
When a stud breaks off, the options start with LEFT HAND drill bits, after some penetrant and, if possible, heat. Then the options widen. Even in steel, dissolving can work well, but it takes care.
Losing a broken extractor or drill from a broken stud is no issue chemically. The broken part is coming out anyway, so why not?
Getting a broken bit out of steel when drilling a hole into a part takes more care, but, if the hole isn’t a critical size (and it isn’t, or you wouldn’t be drilling) and can handle being a few tens or hundreds of microns over, then dissolution is fine, as you don’t need to dissolve the whole thing. Just enough to loosen it. Then water flush and get the bit out. Hypo needles help, as small gauge needles will get down the flutes to but the active where it is needed, as well as flush it out. Flush hard. Dilution is the solution to stop dissolution.
As a side note, for those with the proper experience and training, nitol works well, as it will preferentially hit the harder steel over softer, as well as over non-ferrous. If you have the background to do it safely, this is enough information. If you haven’t been properly trained to handle nitric acid and derived solutions, then this is not for you, as permanent disfigurement or death are not worth it to get out a broken drill it. Stick with the alum.
Of course, there is always a diamond point in a die grinder or dental drill, which are quite effective.
Oddly enough, I am, among other things, a trained watchmaker. I can attest that this happens to both us and clockmakers in brass often enough that we use stuff like this to get bits and broaches out.
I’ll admit- I’d never heard of this particular method though. I’ll have to do some research. I was taught heated vinegar, iodine, and bleach in that order of strength to remove broken bits, and have used bleach- it’s aggressive, but it works.
Yet again, I learn something new from HaD
If the thing the bolt is stuck in is made of aluminum, he could used gallium to get the bit out. Oh, wait… backwards of the desired result…
Only if the broken bolt is more important that the thing it’s stuck in…
Found a Practical Machinist thread on the subject: http://www.practicalmachinist.com/vb/general/broken-tap-removal-chemical-methods-198135/
Useful notes from another post:
“You can dissolve ferrous metals out of non-ferrous metals (such as aluminum, bronze, etc.) with alum (potassium aluminum sulfate). It’s a sour tasting pickling spice sold at grocery stores (try your local food co-op) that helps crisp pickled vegetables.
Submerge the offending part into a pyrex dish (you don’t want to do this in a steel pot!!!) full of a solution of 4tbsp alum to 1 cup water (as much as you need to completely cover the bolt hole), I then placed the pyrex dish into a pot full of nearly boiling water and let it go. Heat is ESSENTIAL, or the alum won’t dissolve. You’ll see the steel part start to bubble a little bit (it’s letting off pure oxygen) as the alum goes to town.
This isn’t the fastest process in the world, it took me about 12 hours before enough of the tap dissolved to get it out. You could probably drill out the center of a bolt to help remove the bulk of the metal and then use the alum to dissolve away the rest leaving perfectly preserved threads. You can also either let it go until the part is completely dissolved, or you can speed things up by breaking little bits off with a sharp pick every hour or so.
The real draw is that there’s relatively minimal labor involved, it’s really cheap (~$3 per application), totally safe and odorless (let the solution get cold and the alum will form into big crystals you can just save or throw away), and really hard to mess up. Plus if your threads aren’t already ruined up, they will be perfectly preserved!”
Can it melt steel beams?
Go outside.
Went outside. What now?
Go play in traffic.
Only if it is soaked in jet fuel.
does anyone know the details of the chemical reactions taking place? (what causes the iron to dissolve, and why the brass doesn’t react (some people use this technique on aluminium also))
The alum oxidizes the metals. Both metals ‘rust’ but for most brasses and aluminums the oxides act as protective layers. For ferrous metals the iron oxides flake off rather than stay bound to the metals underneath.
I used 70% nitric acid to very slowly remove a broken stud in a small engine case.
Wish I knew about this alternative, but I’m not sure how I would have kept it hot.
You could have used a clay dam and a heat gun or an infrared space heater.
I used this method to dissolve a tap I broke off in an aluminum rail a few months back. Worked like a charm!
I’m doing this on my Harley. I don’t have a heat source though and it’s in a PVC pipe with hydrogen peroxide. Temps get about 100 degrees or so. Think it will work?
Never knew this one.
Old safes used alum & sawdust as the fire resisting part. When they got hot the alum would sweat water into the sawdust insulation and stop it from burning. Evaporation kept it all cool enough that chickens inside could survive a bonfire.
I guess the rust issue is why they switched to passive insulation.
Did something like this once with a busted VW exhaust stud. Made a dam with plasticine, filled it with a bit of battery acid and waited a few days. After that the hardened steel drilled out like butter.
As I understood it, the acid attacks the carbon in the steel, turning it into soft porous iron.
Oh where’s my memory. It wasn’t the stud that was the problem — it was the broken ‘easy out’.
There are two kinds of crystal mentioned: “ammonium aluminum sulfate” and “potassium aluminum sulfate”. will both of them work?
What about the other alum types?
https://en.wikipedia.org/wiki/Alum#Types
Types:
5.1 Potassium alum
5.2 Sodium alum
5.3 Ammonium alum
5.4 Chrome alum
5.5 Selenate-containing alums
5.6 Aluminium sulfate
Hi everyone, I am working on a heat-sink for a laptop (trying to make it cool passively). I was stupid enough to brake my tap and I am soaking the heat-sink in a solution of alum as we speak. It has been about 48 hours and today I tried to fracture the left-over bit. No results thus far. I am containing everything in a plastic tub (presumably poly-ethylene) and started with hot tap water.
My questions are:
1) Is it normal for it to take that long (its a really small tap, M3)
2) How important is it to heat the solution? My current container doesn’t allow it to be cooked on a stove or similar.
Thanks for your time!
Regards
Pieter
A heat sink for a laptop sounds pretty small. Why not try a stainless steel cooking pot as the vessel and heat it. If you do not see bubbles, its not working.
It is going to attack the pot!
although this thread is about removing steel items from non-ferrous metals with chemical means, there are a couple of alternative methods that work well too. If it’s broken off flush, welding a tip of an old screwdriver to it at a good angle to use as a handle to spin it out can work wonders. The heat from the weld is key. also placing a small nut on top and filling the center with weld can work too.