While debugging a strange battery failure in a manufacturing process, [Josh] discovered a new (to us) LiPo battery failure mode.
Different battery chemistries react differently to temperature. We’ve used lithium exclusively in high-altitude ballooning, for instance, because of their decent performance when cold. Lithium batteries generally don’t like high temperatures, on the other hand, but besides the risk of bursting into flames, we had no idea that heat could kill them. When the battery’s voltage is already low, though, it turns out it can.
[Josh]’s process required molding plastic with the battery inside, and this meant heating the batteries up. After the fact, he noticed an unreasonably high failure rate in the batteries, and decided to test them out. He put the batteries, each in a different initial charge, into a plastic bag and tortured them all with ice and fire. (OK, boiling water.)
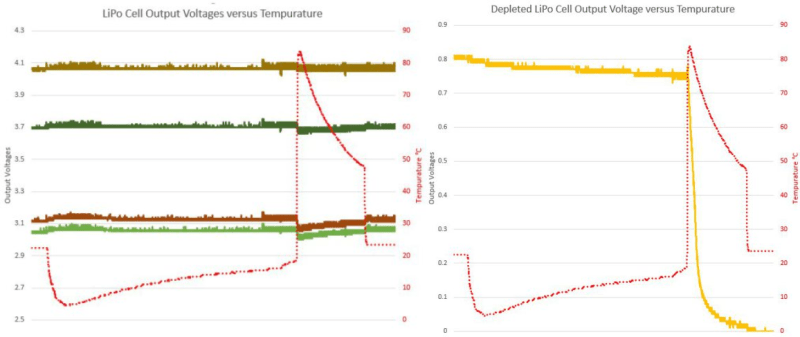
When the batteries got hot, their voltage sagged a little bit, but they recovered afterwards. And while the voltage sagged a little bit more for the batteries with lower initial charge, that’s nothing compared to the complete failure of the battery that entered the hot water with under 1V on it — see they yellow line in the graphs.
There’s a million ways to kill a battery, and lithium batteries are known not to like being completely discharged, but it looks like the combination of deep discharge and heat is entirely deadly. Now you know.














That’s is 100% correct as leaving a cell at low voltage will definitely kill it if left long enough.
“that’s nothing compared to the complete failure of the battery that entered the hot water with under 1V on it”
Lithium cells are never supposed to be discharged below 2.5 Volts because they become chemically unstable. The battery was already a dead man walking when it was discharged that low, and the heat simply accelerated the damage.
I have seen the effect on smartphones and gps’s that stood in the sun.
I have also see this on dash cams that have LiPos.
There’s not as much risk of that happening here in the UK compared to lots of other countries :)
The Mobius camera has an option of replacing the lipo with a super capacitors for such situations, which many owners in hot climates praise.
And… it turns out that “long enough” is very, very short above 75 deg.
With the temperature rise being so fast, (pouring boiling water into the pot), the exact threshold temperature cannot be determined from this experiment.
There are two bad things for lithium batteries. High temperatures and undervoltage. Combine them and apparently that kills them. Duh!
I don’t see the value of testing a lipo for heat-treatment that has 0.8V of “already completely mistreated”. (I doubt he followed the correct procedure to charge the fully depleted battery: max 0.05C charge current).
I thought this result was actually the most interesting of all! The fact that a depleted cell would very quickly loose all of its resting voltage a critical temperature that is below its spec’ed maximum operating temperature, and that it would not recover after returning to ambient is not at all obvious to me and very different than how competent batteries seem to react If this behavior is predicted, please explain the reasoning so I don’t ever have to boil batteries again! :)
I actually don’t think the temperature is what killed your battery but rather the complete discharge. The temperature seems to induce a loss of charge on each battery. The weaker the battery the greater this loss of charge.
That it dropped to zero can be explained by the discharge characteristics of a Lithium battery. Over it’s charge life the voltage drops slowly until it gets towards the end of life where it suddenly plunges. You started with a battery that was already beyond end of life which would have had very little charge in it and then did something that is shown to reduce the charge across your entire sample of batteries.
If you want to confirm this you need a control. Take all the batteries at the same charge level as your boiling experiment and hook them up to their rated discharge load. When you’re done I’m willing to bet that the battery with an initial voltage of 0.8V is also completely dead.
The battery wasn’t “end of life” but simply a battery that was mostly discharged. It was the initial, low, charge on the battery combined with the high heat that killed it.
‘Duh’ is a bit harsh. Not everyone who reads HaD is an EE specializing in battery chemistry.
The graph here is very useful: http://www.mpoweruk.com/lithium_failures.htm
That is brilliant, thanks for sharing.
Very helpful, but I think this (like most of the LiPo C/V data I found) applies to *operating* conditions rather than to open-circuit temporary exposure conditions – which is what I was interested in.
1 volt? Aren’t they irreversibly damaged below 3 volts?
Depending on the specific lithium-based chemistry (some are safe down to 2.7v), yes, below their low voltage cutoff, the energy you are taking from the cell is coming from an irreversible chemical reaction, i.e. a reaction that cannot be undone by putting current into the cell’s cathode.
The threshold is not “3V” but depends on the specific battery and can be as low as 2.5V.
Second: If you accidentally discharge below the threshold, and quickly charge the battery again using the proper procedure, the battery can usually be recovered for more than 90% of its remaining capacity. Your capacity will take a “sudden” hit, but if you accept the battery having a slightly smaller capacity you’ll be fine.
The “proper procedure” is to charge it really really slowly until the voltage reaches at least 3.0V.
There’s a procedure I saw on Youtube, for a lithium phone battery that won’t charge, after being left flat for too long. Basically you zap it with 5V from a stripped USB lead. Bit unsubtle, but it worked on my watch-phone’s battery. Only takes a few seconds, just to get the juices flowing.
That method will destroy a phone battery’s internal fuse and render it unusable.
Tried it on standard Nokia cells back in the day – killed every single one.
I always used some power source and some resistance to push a few mA through it for some minutes. Then in the phone to charge. Most improvised i did was some modem adapter, aluminium foil and potato as resistor…. for that moment when a friend casually tells you his phone is dead and not charging.
Worked for my watch-phone’s shitty battery. Just a couple of seconds. Not sure it has a fuse, and even so should be able to take the current a USB adaptor can put out, that’s going to be the standard charging current anyway, so fuse should be OK. Even with 5V versus the proper 4ish volts.
Of course on Youtube you get people who think this is how they’re gonna charge their batteries forever now, throw away the charger! But since the battery was dead, dead, dead, didn’t have much to lose.
Wait are you sure that isn’t a Ni-Cd cell? there is a capacitor method for clearing those that is quite similar(it destroys crystaline structures that form shorts by surging them). I would not suggest doing a ‘spike’ to any li-ion/poly cell TBH. charge them up slow and steady. Also I’m sure any with a protection pack will just the spike once it goes over a threshold voltage.
USB doesn’t supply anywhere enough current to “blow whiskers”, as was done with NiCads. That was typically done with a capacitive discharge, tens of amps peak. Unnegotiated USB is 500mA max, maybe less, I forget exactly how that works.
It’s possible that brief exposure to a voltage higher than any normal charging voltage forces current past the protection circuit’s undervoltage lockout, which prevents you from recharging the battery once the voltage drops past a point generally considered safe. So if the battery is just a little below that point, that brief forced charge can raise it past that point, and reset the lockout. At least with some circuits, as [Dax]’s experience shows.
I haven’t heard this method before. But I have heard of physically bypassing the protection circuit, and giving the battery a bit of charge directly, to accomplish the desired result.
Definitely lithium. And I think Chris C’s got it, the voltage had fallen so low (been a long while since I’d used it), that the undervoltage thing had cut the battery off, so no way of charging it, at least not within the phone. I think the 5V gave it a kick to put it over the threshold, and after that it started behaving properly.
I know it’s a bit drastic, but battery was doing bugger-all otherwise, it was that or throw it away. Quite pleased it worked!
I have to agree with the above. Your battery is already damaged when it’s below it’s cutoff voltage. Heating the battery to further test its recovery is moot. If he wants to increase the longevity of his batteries, he needs to make sure the batteries are charged up at least to 60% of their capacity.
While this was a completely pointless exercise as others have mention Li-Ion cells are done when you have taken too many lithium ions out of the anode, or allowed too many to intercalate in the cathode, meaning the voltage potential can never be restored through electrolysis.
It was however a good demonstration of the completely sensitive and antiquated technology that is lithium ion.
It is worth noting that battery chemistries are a closely guarded secret (as far as dendrite protecting, or SEI layer additives are concerned) and that ion concentrations are very sensitive to temperature, and that liquid electrolytes are prone to fire. It really is just a matter of time until this technology goes the way of the dodo.
Not many thing come close in energy density to lipo batteries.
Though LiFePO4 and silver zinc batteries come pretty close and are much safer.
and I cant wait for them to come down in price because their longevity is dramatically better, they take way WAY more charging abuse and honestly are a far superior battery to LiPo.
I have charged LifePO4 batteries at 2X rated amps for 1 hour past full charge with no damage. it’s why they are now used in race cars and racing motorcycles as you can get a lead acid durability and capacity in 1/10th the weight
Lead acid isn’t renowned for either durability or capacity.
The durability of LiFePO4 batteries was overhyped. They were much more sensitive to operating temperature than many LiIon formulations. Maybe, after much disappointment, its finally reaching what was promised, but its hard to tell, since so many people swallowed the initial hype.
SR44 coin cells are recgargeable?
That’s siver oxide, but silver-zinc is indeed a secondary cell (rechargeable)
I’m sure it is just a matter of time before lithium ion batteries are obsoleted, but that isn’t saying much. The question is how long? Given that there don’t seem to be other chemistries making inroads, I think LiIon is going to be with us for some time longer.
charging can cause fires too take the hoverboard like scooters in recent weeks they fired up while charging
Correct term is “fake hoverboard” or “so-called hoverboard”. Damn Chinese spivs taking advantage of Back To The Future!
From [Josh]’s FAQ:
“I am working an a problem were a device with a LiPo battery is subjected to a high temperature during a molding process during manufacture. These devices started failing QA at a slightly higher rate after the temperature of the molding process was increased. One theory was that the exposure to the higher temperature might have caused marginal batteries to drop enough voltage to fail testing after the molding step was complete.”
This begs the question, why was this manufacturer using “marginal” batteries in the first place? From what I can tell, the affected cells weren’t even marginal, they were clearly overdischarged, and inherently unsafe. Testing the batteries and discarding these should have been done first, as part of the most basic QA, before molding them into a product where they are presumably no longer replaceable. Who is this manufacturer? I’d like to avoid their products!
“I looked for data on the voltage response of LiPo’s to non-destructive temperature extremes and could not find anything at all. (There is lots and lots of data on battery lifetime while being operated in extremes, however!)”
Consider a battery always in operation, even if there is no external load. I’ve seen some data showing lifetime at various temperatures and states of charge. Lifetime drops exponentially as either temp/SOC approaches extremes, much less both simultaneously. Li chemistry is most stable at 50% charge, and should ideally be kept close to that when temperatures are high, not near 100%, and definitely not 0%. (For long term storage, 50% charge and temp just above freezing, slows self-degradation to almost nothing.)
“I started with Mathematica… I gave up… Then I switched to MATLAB… I hit a wall… So as a last resort, I fired up Excel. Excel lumbered and creaked, but ultimately it (slowly) let me add my secondary axis and put the data sets on and even point-and-click color everything. This experience left me broken. I want to hate Microsoft like everyone else…”
So [Josh] wasted hours trying to use tools meant for hardcore mathematical analysis, with their corresponding complexity, to make a simple graph because of… peer pressure? I hate Microsoft too, but I don’t hate myself and am not a masochist. If I need a graph I fire up Excel without hesitation, it’s easy and gets the job done. If that wasn’t an option for any reason, I’d download and try OpenOffice Calc, long before Mathematica or MATLAB.
This would have been a good FoTW.
All excellent questions and analysis. Your analysis – it’s what I would have concluded had I been smart enough. I have found (as you) that usually the simplest tool can provide a quick result, just due to sheer setup time. If you go back to the days of SuperCalc and VisiCalc and others – Excel is a walk in the park in comparison, so I don’t consider it lacking unless of course it is not capable of rendering the result. On the battery part, I would guess that similar battery categories from different manufacturers would result in a wide range of results due to internal structure and different chemistry, but in general I agree with your 50% conclusion. Expanding that graph to include those comparisons between manufacturer would be interesting, especially if your projects are at the extreme on the temperature and duty cycle scale. But I damn sure don’t have the time or money to do so. Project for another day, a really boring day.
I did not mean to suggest that the manufacturer could be intentionally procuring marginal batteries. They are not.* The batteries are sampled when they come in the door and each and every battery that comes into the molding process has already passed at least one QA voltage test before entering the molding process.
So, knowing that the batteries had tested good at some point before the molding process, and that they failed at some point after, it is a valid (but not necessarily likely) theory that…
IF
(a) marginal batteries were entering the molding process
somehow (lots of way this could happen!)
AND
(b) temporarily exposing a marginal battery to a higher temperature could
cause the battery to fail a voltage-based QA after it returned to ambient,
THEN
the new higher process temperature could potentially be responsible for
increased failure rate.
As Hack-A-Day readers, we all know our Boolean algebra – if you can show that either input to an AND is false then you know the output is false without having to evaluate the other input.
To evaluate (a) would have required testing the resting voltage of every single battery on the line just prior to entering the molding process.
To evaluate (b) would have required boiling some batteries on my stove in my apartment.
Injecting a new per-unit step on running production line that is 12 time zones away is hard.
Boiling some batteries on my stove is
easyharder than I expected, but still not so hard.Evaluating (b) seemed like the right way to go.
So the question is: Can exposing a marginal battery to a higher process temperature have the effect of lowering the output voltage enough to fail a subsequent QA test at ambient?
I did not know the answer to this question. I could not find any data that answered the question. I looked hard. Even the battery manufacturer did not have data on this case.
So I did the experiment. I published the results to hopefully save the next person who has this question a few steps.
To be clear: this experiment had a _negative_ result (the increase in the process temperature would likely _not_ have caused marginal batteries to fail the subsequent QA test), but negative results can be just as important as positive ones. In this case, the negative result allowed us to reject this theory as very unlikely and focus efforts on the remaining ones.
*It would be nice to just order the manufacturer to never, ever allow any out-of-spec parts in any step of the line and then you would never ever have to consider any failure mode caused by them. Unfortunately, if you order enough of a part then you will get marginal units. Parts can also go out-of-spec as a result of the manufacturing processes they go though, so even if you could 100% pre-qualify everything when it comes in the door, you still have to deal with marginal components on the line. The problems of making the first of something are different than the problems of making lots more of them!
In my (limited) experience, there is a difference in loaded versus unloaded LiPo battery response to environmental exposure. If you have found otherwise, please share links! Thanks!
To clarify, I *love* Mathematica. I think it is the simplest and fastest way for doing
everyalmost every kind of plot I’ve ever thrown at it. I mentioned the whole episode because making a double-Y plot turned out to be so unexpectedly hard. Compare the linked 2-Y plot code to doing a single YListPlot[]. Don’t you think it is surprisingly complicated and un-idiomatic? If there is a better (simpler, more idiomatic) way to do a chart like this in Mathematica, please do tell! I honestly want to know! Thanks!Re: “So the question is: Can exposing a marginal battery to a higher process temperature have the effect of lowering the output voltage enough to fail a subsequent QA test at ambient?”
You did conclusively find that at SoC <90%, a small voltage drop occurs at high temperature; and that might be useful information. But I think that for purposes of solving the manufacturing mystery, maybe you've asked the wrong question, and used the wrong test methodology.
The question in my opinion would be, does brief exposure to extreme temperature accelerate the chemical degradation of a LiPo battery at low SoC, enough that lifetime is substantially affected?
Determining that would require measuring the capacity of the cell both before boiling, and after return to ambient, which appears not to have been done. If a substantial loss occurs, the manufacturer really ought not to be doing that, regardless of whether the majority of the batteries still function enough to pass a quick QA check.
You tested a cell starting at 3.05V, and a cell starting at 0.8V, but I see nothing in between. This does, even if it's unintentional, give the appearance that the manufacturer is using cells as low as 0.8V. It really isn't a fair test either. I know it's tempting to exaggerate parameters in order to get a test to display an issue that normally only occurs a fraction of the time, but that can mislead if taken too far. (Favorite example: A study concluded that a particular artificial sweetener causes cancer. But to get results in a reasonable amount of time, the test rats were fed such a huge amount that they could not eliminate it fast enough; and it was forming crystals in their urethra. The only cancers observed were urethral cancer. Is the sweetener really a carcinogen?)
So what is the lowest real-world voltage at which these cells enter the molding process? Take that and test one cell at 0.2V lower, and another at 0.4V lower. You may not get any clear and immediate failures from that test, unlike the cell tested at 0.8V. But capacity testing will still reveal if the damage mechanism, which could lead to failure, is proceeding at a significant rate.
Re: "In my (limited) experience, there is a difference in loaded versus unloaded LiPo battery response to environmental exposure. If you have found otherwise, please share links! Thanks!"
That's correct, the internal resistance does change. The intent of my "consider a battery always in operation" was to point out while an unloaded battery may as a whole be described as in a homeostatic condition, there is still loads of activity. Molecules being added and removed from crystals, ions swapping, etc. The lifetime vs. temperature data you found is quite relevant.
Re: "I mentioned the whole episode because making a double-Y plot turned out to be so unexpectedly hard. Compare the linked 2-Y plot code to doing a single Y ListPlot[]. Don’t you think it is surprisingly complicated and un-idiomatic?"
Yes, it is. Have used plenty of mature tools that offer incredible power, yet require incredibly hackish approaches when trying to accomplish something that should be simple – and common! Makes you wonder what the developers are thinking. I have better luck starting with simpler tools for infrequent tasks, just enough to get the job done, then working my way up the chain if necessary rather than the reverse. Sorry, I can offer no advice specific to Mathematica or MATLAB. Being a programmer has so far saved me from needing those tools.
One of my favorite batteries is the Energizer L91 — http://data.energizer.com/PDFs/l91.pdf
Alas, Not rechargeable. Lithium/Iron Disulfide (Li/FeS2).
Strangely enough, some lithium phosphates I used actually recovered if heated up after an extreme discharge (1V), without heating they refused to charge at all.
I know that Li-ion do not work very well if cooled below 0C as the ions do not intercalate correctly and form lithium metal which can degenerate into dendrites on the next charge and cause vent-with-flame.
Title is missing “Fail of the week: “
Despite all the bashing, thanks for the reminder on heat and LiPos! Good to know that one should be careful.
Despite all the hand holding you may see elsewhere, this is hackaday not kumbayah a day. It’s not bashing either it’s the obvious, Hackaday really needs a chemist on staff.
Just because a thing is obvious to one person does not mean it is obvious to another.
And a funny thing about the obvious; often it turns out to not be as obvious as one might think.
Even “obvious” concepts need testing and validation. Otherwise they are just assumptions. And you know what assuming leads to…
That battery was already “dead” at 1V before it ever got wet.