How does one go about measuring the mass of an object? Mass is defined as the amount of matter an object contains. This is very different from weight, of course, as the mass of our object would remain the same despite the presence or size of a gravitational field. It is safe to say, however, that most laboratory measurement systems are here on Earth, and we can use the Earth’s gravity to aid in our mass measurement. One way is to use a balance and a known amount of mass. Simply place our object on one side of the balance, and keep adding known amounts of mass to the other side until the balance is balanced.
But what if our object is very small…too small to see and too light to measure with gravity? How does one measure the mass of single atom? Furthermore, how does one determine how much of an object consists of a particular type of atom? There are two commonly used tools just for this purpose. Chances are you’ve heard of one of these but not the other. These tools used to measure substances on the atomic level is the focus of today’s article.
Qualitative vs Quantitative
If Sir Isaac Newton is knocking on your left cerebral cortex right about now, you’re thinking in the right direction. Force is equal to mass times acceleration. If we apply a known force to our atom and measure its acceleration, we can know its mass. But first, we must define two techniques that often get confused with measurement systems on the atomic scale.
Qualitative:
A qualitative measurement will tell you what is there, but not how much of what is there. It’s subjective in nature, but useful in understanding certain situations. Saying “There are a lot of Arduinos on your workbench” would be a qualitative analysis.
Quantitative:
This is the more intuitive measurement. It’s based on numbers, and is objective. Counting the precise number of Arduinos on your work bench is a quantitative measurement.
These terms are used in other fields and have various definitions that apply to each. But for the purpose of this article, understand that a qualitative measurement will tell you “We have Arduinos here” and a quantitative measurement will tell you “We have X number of Arduinos here.” See the wiki for a more technical explanation of qualitative vs quantitative measurement when applied to chemistry studies.
Mass Spectrometer
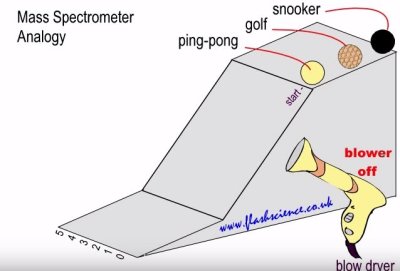
I blew past the diagram above without mentioning it, but glance back for a moment. Let us imagine a small ramp and a handful of small balls that will roll down the ramp. Each ball has a different mass. Now let us imagine that we take a blow dryer and blow the balls sideways as they roll down the ramp. The balls with the most mass will be deflected least, while the balls with the least mass will be deflected most. By looking at where the balls land at the end of the ramp, we can measure the mass of the balls.
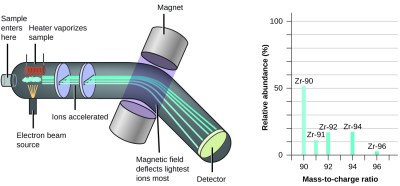
A mass spectrometer measures the mass of a single atom or molecule by replacing the ramp with an electric field and the blow dryer with a magnet. First the atoms must be heated to a gas state. Then they are bombarded with high speed electrons to knock electrons free from the sample gas. This turns the sample into positive ions. Note that electrons weigh 1/1836 amu, so missing electrons will not have any measurable effect on the mass of our sample.
Now that the sample has a positive charge, we can use an electric field to accelerate them! Two “plates” at different potentials are used to focus and accelerate the sample ions toward a magnet. The ions are attracted to the magnet, and become deflected by the magnetic field. The amount of this deflection is related to the mass of the sample ion.
Just like how we measured where the balls hit at the bottom of the ramp, we measure where the ions hit at the end of their journey. By measuring where they strike the “detection screen”, we measure the amount the ions were defected by the magnet, and therefore the mass of the ion.
High Performance Liquid Chromatography
While a mass spectrometer can give us the mass of atoms and molecules, it is not usually used to quantify how much of an element or molecule is contained within a sample. For this reason, mass spectrometry is generally considered a qualitative measurement.
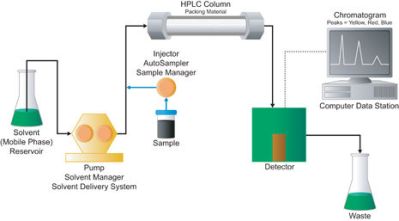
To know how much of an element or molecule you have, you need to use a technique called Liquid Chromatography. LC instruments that operate under pressure are called HPLCs (High Performance Liquid Chromatography). Often, mass spectrometers are combined with HPLCs and are called LC-MS machines.
Just how does an HPLC tell us the amount of an element or molecule in a sample? Let us imagine that we have a bucket of nuts and bolts of various sizes. Our goal is to separate and group them according to their individual characteristics. One way of doing this is with a “friction filter.” Imagine we dump the bucket of nuts and bolts into our filter. At the bottom of the filter output is a conveyor belt. The smaller nuts and bolts experience less friction as they fall through the filter and come out first, while the larger ones experience more friction and come out last.
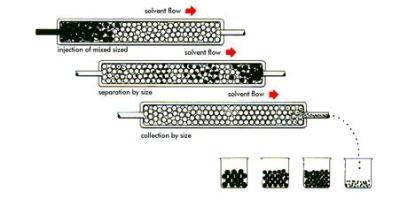
An HPLC does something similar to molecules. A sample is dissolved into a liquid. The liquid sample is then forced through what is known as a column under high pressure. The column is somewhat analogous to our friction filter. The size and makeup of HPLC columns vary with the approximate size of the sample that needs analyzing. But they all work the same way. The high pressure forces the sample through the column, and interactions with the column packing (not friction) causes different sized molecules to move through the column at different rates.
With the combination of a mass spectrometer and an HPLC are used to acquire a quantitative measurement of molecules. The mass spectrometer lets scientists know what substances a sample is made of, and the HPLC is then used to measure how much of each particular substance is contained within the sample. The techniques are applied in a wide variety of fields. The accuracy of this method allows for measurements of pesticides (and everything else like vitamins or hormones) in food, and is also invaluable in developing and testing pharmaceuticals.
















Wow Will, your description of HPLC only covers one type (size exclusion) and it’s the larger molecules that come out first. Other types of HPLC use the differential binding affinity of molecules to the material in the column, not the size of the molecules. In neither case is friction involved.
Yeah, friction is not the right word. I’ll make some edits. Thanks for the constructive comment!
I’m still left wondering how to measure the mass of an atom…
Dip a filament in a solution of something, then let it dry.
Heat the filament in a vacuum and the atoms from the solution will boil off. If there’s a high-voltage plate further away, the atoms will pick up a charge and fly towards the plate.
Put that ion stream through a magnetic field, and the charged ions will be deflected. The plate can be moveable, and you adjust it to get the maximum current.
Knowing the velocity of the ions from the electric field, and the strength of the magnetic field, you can calculate the amount of deflection proportional to the mass of the ion.
The deflection you measure indirectly tells you the mass of the ion.
“… it [mass spectrometer] cannot quantify how much of an element or molecule is contained within a sample.”
I’d say mass spectrometer can very well determine quantitatively the amount of specific ions. That is what ICP-MS is used for. Even the image from Khan academy has “relative abundance” in the chart…
Fine. I changed it from “cannot” to “not usually used to…”
I’m with Will’s original interpretation on that one. What you get from a mass spec is a fragmentation pattern. You find out the amount of each ion detected, but that depends on how stable the ion is as well as how much there is in the source and how those ions are produced which depends on the ionising current and lots of other things. Isotopes do not affect the process much, so isotopic abundance plays a big role in the resulting pattern and it’s interpretation. That also means that quantitative work is possible on isotopes. It is possible to do comparative stuff between samples. I’m not pushing for it to be changed back, there is room for interpretation, but in it’s original form it looked fine to me. Assuming you know what the sample is or can work it out, a single run from a mass spec still tells you nothing about the elemental or molecular abundance, HPLC does, because the molecular absorbance measured by it’s detector is fixed.
What about inorganic compounds and the different types of ionisation and also the different types of mass analysers. Not a bad article though….just lacking some of the finer points. I used to work for Waters Micromass….it was my first job after university!
HPLC pretty universally is High Performance Liquid Chromatography. The wiki says it used to be Pressure, but this is news to me and must be more than 2 decades ago.
Fixed. THanks!
The weird thing is, no one really talks about low-performance liquid chromotography (don’t they just call this analytic, as opposed to preparative?)… but they probably would be more likely to talk about low-pressure liquid chromatography (spin-columns, gravity fed, water-faucet vacuum, heck vacuum in general is ‘low’ pressure). How else can we change the industry other than one article at a time and swaying editors!
Nice intro to one aspect of metrology. Metrology underlies pretty much all science and engineering, data collection or process control. You could do an entire series on just that one topic.
I totally agree with previous comments. Nice article, but some nuances are needed.
The purpose of a liquid chromatograph is mainly for separating mixtures.
A mass spectrometer can have problems if complex mixtures are introduced at once, hence the coupling with a separation device.
The major parts should also be mentioned: ionization (generating ions) and analyzers (mass separation).
And finally: a mass spectrometer not only records the masses of the ions, but also the amount, so the instrument can be both qualitative and quantitative.
It is very hard to explain the whole picture in one page, it takes me 10 lessons and wiki about twenty pages.
It has been my experience that most labs will use a mass spec for identification and LC for quantification. For instance, let us suppose I have a sample containing two proteins. I will use a mass spec to ID the two proteins. Then I would use that data to set up my LC to see the ratio.
Metrology, not meteorology. Why is there a drawing of a TV weather announcer / comedian?
Because the staff wants to get paid?
I think that’s supposed to be Bill Nye, the Science Guy.
That’s the first thing I thought.
Though, Bill Nye isn’t really a science guy anymore. Former TV show host that now complains about gasoline and global warming all day.
Bill Nye was always more of an engineering guy than a science guy…
OK. Two more items for you. First: I don’t know what “Mass is defined as the amount of matter an object contains” means but mass is defined in terms of inertia. When you use a scale you make the assumption that inertial mass is the same as gravitational mass, but no one has proven this to be true. Second: the ions are not attracted to the magnet. They experience a force at right angles to the magnetic field and to their path (The right hand rule for vectors). The arc is in a plane perpendicular to the magnetic field. The ions do not get closer or further away from the magnets. In fact, the magnetic field does no work on the ions. The ion’s velocity vector changes direction but not magnitude. The force is a cross product and work is force times distance. The ion motion is always at right angles to the instantaneous force and therefore, force times distance is zero.
http://antoine.frostburg.edu/chem/senese/101/atoms/faq/how-does-mass-spec-work.shtml
nice animation
It was written: “When you use a scale you make the assumption that inertial mass is the same as gravitational mass, but no one has proven this to be true.”, but that assumption is the core assumption of Einstein’s General Relativity which makes it as “proven” as any part of physics.