One of the most widely recognised product brands in the world is probably Coca-Cola, and its formula is famously kept a secret through precautions that probably rival those of many nation states. There are other colas, and there are many amateurs who have tried to copy Coke’s flavour, but in well over a century, nobody has managed it. Why does [LabCoatz] think his attempt will be successful where others failed? He has friends with their own mass spectrometers.
mass spectrometer6 Articles
Mass Spectrometer Tear Down
If you have ever thought, “I wish I could have a mass spectrometer at home,” then we aren’t very surprised you are reading Hackaday. [Thomas Scherrer] somehow acquired a broken Brucker Microflex LT Mass Spectrometer, and while it was clearly not working, it promised to be a fun teardown, as you can see in the first part of the video below.
Inside are lasers and definitely some high voltages floating around. This appears to be an industrial unit, but it has a great design for service. Many of the panels are removable without tools.
Uranium-241 Isotope Created And Examined Via Multinucleon Transfer Reactions And Mass Spectrometry
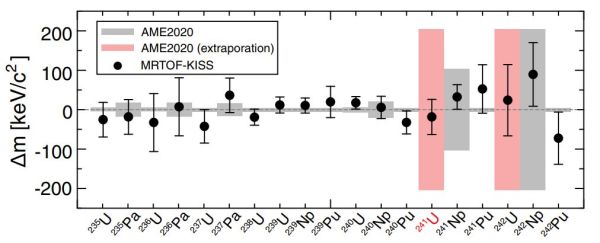
A recent paper (PDF) in Physical Review Letters by T. Niwase and colleagues covers a fascinating new way to both create and effectively examine isotopes by employing a cyclotron and a mass spectrograph. In the paper, they describe the process of multinucleon transfer (MNT) and analysis at the recently commissioned KEK Isotope Separation System (KISS), located at the RIKEN Nishina Center in Japan.
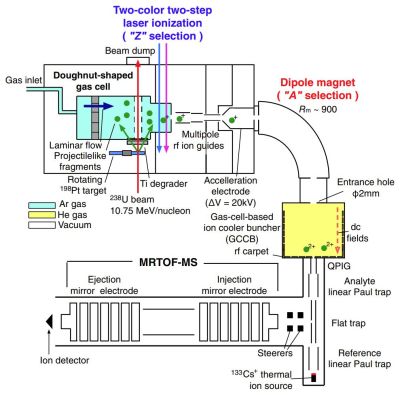
yellow-colored areas are filled with Ar and He gases, respectively. Differential pumping systems are located after the doughnut-shaped gas cell as well as before and after the GCCB. (Credit: Niwase et al., 2023)
The basic process which involves the RIKEN Ring Cyclotron, which was loaded for this particular experiment with Uranium-238 isotope. Over the course of four days, 238U particles impinged on a 198Pt target, after which the resulting projectile-like fragments (PLF) were led through the separation system (see sketch). This prepared the thus created ions to be injected into the multi-reflection time-of-flight mass spectrograph (MRTOF MS), which is a newly installed and highly refined mass spectrograph which was also recently installed at the facility.
Using this method, the researchers were able to establish that during the MNT process in the cyclotron, the transfer of nucleons from the collisions had resulted in the production of 241U as well as 242U. Although the former had not previously been produced in an experimental setting, the mass of 242U had not been accurately determined. During this experiment, the two uranium as well as neptunium and other isotopes were led through the MRTOF MS instrument, allowing for the accurate measurement of the characteristics of each isotope.
The relevance of producing new artificial isotopes of uranium lies not so much in the production of these, but rather in how producing these atoms allows us to experimentally confirm theoretical predictions and extrapolations from previous data. This may one day lead us to amazing discoveries such as the famously predicted island of stability, with superheavy, stable elements with as of yet unknown properties.
Even if such astounding discoveries are not in the future for theoretical particle physics, merely having another great tool like MNT to ease the burden of experimental verification would seem to be more than worth it.
This Homemade Mass Spectrometer Works
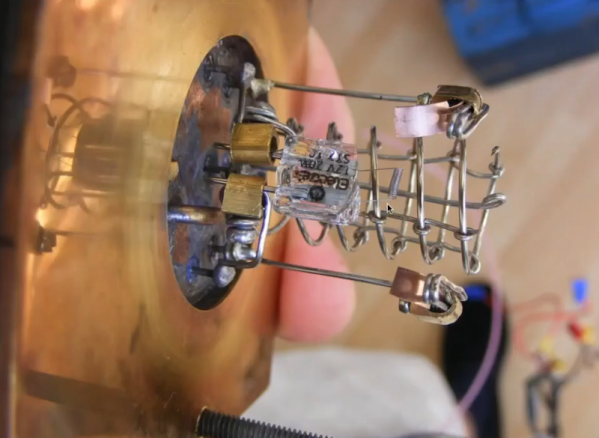
Hats off to [Paul Brooking] as he shows off his homemade mass spectrometer in two recent videos you can see below. The first video demonstrates that the device works. The second video shows details about how it was made.
It’s not a good starter project, requiring quite a bit of sophisticated gear including two-stage vacuum pumps, Peltier cold plates, and ion sources, but if you aren’t familiar with mass spectrometers the basic idea is simple enough. You take a sample and bombard it with electrons. This creates a stream of ions of the component parts of the sample. Ions of heavy elements, obviously, weigh more than ions of lighter elements. A magnetic field deflects the ions, and the lighter ones are deflected more than the heavier ones. By detecting ions at a certain spot in the deflected beam, you can determine the relative amount of ions at a certain mass.
[Ben Krasnow] Builds A Mass Spectrometer
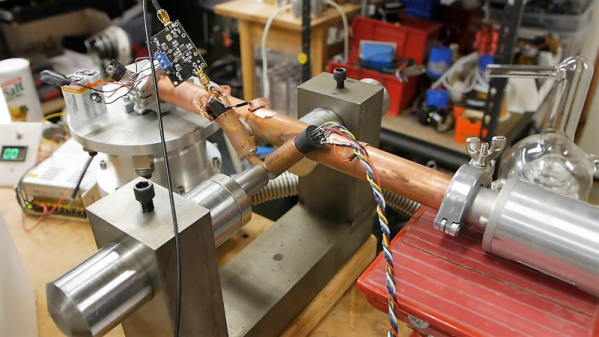
One of the features that made Scientific American magazine great was a column called “The Amateur Scientist.” Every month, readers were treated to experiments that could be done at home, or some scientific apparatus that could be built on the cheap. Luckily, [Ben Krasnow]’s fans remember the series and urged him to tackle a build from it: a DIY mass spectrometer. (Video, embedded below the break.)
[Ben] just released the video below showing early experiments with a copper tube contraption that was five months in the making; it turns out that analytical particle physics isn’t as easy as it sounds. The idea behind mas spectrometry is to ionize a sample, accelerate the ions as they pass through a magnetic field, and measure the deflection of the particles as a function of their mass-to-charge ratio. But as [Ben] discovered, the details of turning a simple principle into a working instrument are extremely non-trivial.
His rig uses filaments extracted from carefully crushed incandescent lamps to ionize samples of potassium iodide chloride; applied to the filament and dried, the salt solution is ionized when the filament is heated. The stream of ions is accelerated by a high-voltage field and streamed through a narrow slit formed by two razor blades. A detector sits orthogonal to the emitter across a powerful magnetic field, with a high-gain trans-impedance amplifier connected. With old analog meters and big variacs, the whole thing has a great mad scientist vibe to it that reminds us a bit of his one-component interferometer setup.
[Ben]’s data from the potassium sample agreed with expected results, and the instrument is almost sensitive enough to discern the difference between two different isotopes of potassium. He promises upgrades to the mass spec in the future, including perhaps laser ionization of the samples. We’re looking forward to that.
How To Measure The Extremely Small: Atomic Mass
How does one go about measuring the mass of an object? Mass is defined as the amount of matter an object contains. This is very different from weight, of course, as the mass of our object would remain the same despite the presence or size of a gravitational field. It is safe to say, however, that most laboratory measurement systems are here on Earth, and we can use the Earth’s gravity to aid in our mass measurement. One way is to use a balance and a known amount of mass. Simply place our object on one side of the balance, and keep adding known amounts of mass to the other side until the balance is balanced.
But what if our object is very small…too small to see and too light to measure with gravity? How does one measure the mass of single atom? Furthermore, how does one determine how much of an object consists of a particular type of atom? There are two commonly used tools just for this purpose. Chances are you’ve heard of one of these but not the other. These tools used to measure substances on the atomic level is the focus of today’s article.
Continue reading “How To Measure The Extremely Small: Atomic Mass”