We all know that it’s not the volts that kill you, it’s the amps. But exactly how many electrons per second are there in an amp? It turns out that nobody really knows. But according to a press release from the US National Institute of Standards and Technology (NIST), that’s all going to change in 2018.
The amp is a “metrological embarrassment” because it’s not defined in terms of any physical constants. Worse, it’s not even potentially measurable, being the “constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 x 10–7 newton per meter of length.” You can’t just order a spool of infinite length and negligible cross-section wire and have it express shipped.
So to quantify the exact number of electrons per second in an amp, the folks at NIST need an electron counter. This device turns out to be a super-cooled, quantum mechanical gate that closes itself once an electron has passed through. Repeatedly re-opening one of these at gigahertz still provides around a picoamp. Current (tee-hee) research is focused on making practical devices that push a bit more juice. Even then, it’s likely that they’ll need to gang 100 of these gates to get even a single microamp. But when they do, they’ll know how many electrons per second have passed through to a few tens of parts per billion. Not too shabby.
We had no idea that the amp was indirectly defined, but now that we do, we’re looking forward to a better standard. Thanks, NIST!
Thanks [CBGB123B] for the tip!

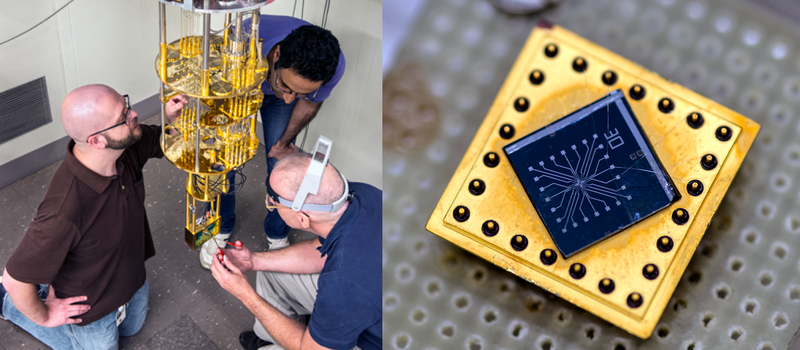














Yes, but…
At least according to the wiki, the charge of the electron (in coulombs) is already known to better than one part per billion – and so the number of electrons in one coulomb is also know to better than one part per billion. How will counting them to a few tens of parts per billion accuracy help?
Government eco-regulations probably.
Actually, the page on the electron says the charge has a “relative standard uncertainty” of 2.2 x 10^-8, so I’d say about 22 parts per billion. But the definition of the Coulomb is the charge transferred by one amp in one second, so the charge on an electron isn’t exactly helpful anyway.
Not sure I follow.
A coulomb is the charge of electron. An amp is being redefined as coulombs/second rather than the arbitrary way it’s currently defined. By counting electrons, they’re piggybacking on the precise (constant, fundamental) values of the coloumb and the second.
They’re not trying to improve the coulomb.
It’s been a long time since we’ve had a really new electron, hasn’t it? What’s with these lazy physicists?
I don’t think that’s strictly true… there hasn’t been a precise/constant/fundamental value for the coloumb, it is merely the amount of charge you get when one ampere flows for one second. So defining the amp in terms of the coloumb would be circular.
What they’re actually doing is redefining the Coloumb to be exactly 1.602E19 electrons, this allows them to redefine the ampere as 1.602E19 electrons per second.
Don’t you mean 6.24E18 Electrons per Coloumb with 1.602E-19 being the number of Coloumbs per electron?
He may not have meant that, but that’s the way it is.
The point is that a Coulomb is not a base unit, an Ampere is. That’s why the Coulomb is defined in terms of the Ampere, not the other way around. We know that a Coulomb is an Ampere-Second (that’s a dash, not a minus sign), but the we don’t have a practical means of ascertaining what an Ampere actually is (until now, apparently)
Interesting article. Isn’t Amps related to Joules?
Quote: “We all know that it’s not the volts that kill you, it’s the amps”
Milli-amps will kill you.
Amps will make you smolder, burn, catch fire and explode.
It’s not the Volts that jolts, it’s the Amps that cramps.
amps * volts * seconds = joules, and they are the ones that really kill.
And you need both all three to kill, without volts amps won’t flow (try grabbing terminals of a car battery which can output 500A), without amps volts are useless (got a static shock recently?), without time nothing will happen either (20mA across the heart takes IIRC 100ms to kill)
Careful there, the static shock can deliver even several amps (that’s why it hurts), what saves you is the time – the discharge doesn’t last very long since such small capacities are involved…
Isn’t that what Mikry is saying? The shorter the discharge time, the less work/energy (i.e. Joules) involved.
As long as one insists on sticking with the dangerous chant It’s not the volts that kills you, it’s the amps and want to be persnickety about it, perhaps one should say it’s not the volts that kill you, it’s the current. Beyond that any “ye old truth” that downplays voltage’s role in the electrocution danger is deadly. A better chant need to be devised.
>We all know that it’s not the volts that kill you, it’s the amps.
reeeee
<sarcasm>I’m crying because he used ‘all’ when he obviously should have used ‘most’.
Together, we will make it through these trying times.</sarcasm>
Finally!
This has bothered me since I first learned Ohm’s law. It’s obvious when you use values that give infinite decimals that there cannot be fractions of an electron. Everyone I asked just told me not to worry about it.
Really looking forward to a proper answer ????
“Everyone I asked just told me not to worry about it.”
In good company! :D
I got the same response when I asked how magnets work!
…and gravity…
…and the Moon…
…and…
…life.
heloooooo?!
42!
But what’s the actual question?
What’s the frequency, Kenneth?
How life works. And we ain’t talkin’ ’bout da birds ‘n’ da bees. Or Benzedrine.
Yep.. as a child I asked those questions so often my mother went out and bought me the “Just Ask!” books. A real answer is always better than lip service. If someone asks a question you don’t know the answer to, find out or point them in the direction of the right person to ask.
????
I had understood that at the small measurements you get weird quantum stuff like partial/probablistic tunneling and the like which can actually almost always leave you with partial charges unless you have set about to build a system like the TFA apparatus which force quantifies each individual electron. I guess the question is if you can get a partial tunneled out electron from an event horizon to produce a a partial Hawking radiation, or if the hawking radiation just averages out over the surface of the even horizon but always in the value of whatever whole tunneled out particle which of cours would measurably change the charge value of the whole singularity only by whole values. Freaking particles vs waves, how do they work?
There’s a typo there matey
ok so how many amps you need to kill by 5 volts?:)
Chest-cavity open or closed? :P
Conductivity of the skin is very important; I’m not sure what the lowest range before danger is.
I’ll bet xkcd has a graph or something!
Sending in a “what-if” question in the form of “if everyone in the world shuffled their feet on carpet and touched you at once, would the static shock kill you?” ….. or something along those lines. I know that there are a ton of variables there, but that’s what makes it fun!
User results may vary….
anything under about ten volts pretty much cant kill(i don’t think it is impossible), skin resistance and breakdown is a linear function below i think 20 volts, so no breakdown cascade can happen.
How about if you had just fell into a tank of molten Gallium first ?
Sure, but don’t try to pierce tour skin ;-)
http://www.darwinawards.com/darwin/darwin1999-50.html
I always thought, the internal resistance of the body (minus the skin) is in the range of 1kOhm. It is the first time I read it could be as low as 100 Ohm. What is the real value?
it isn’t quite as linear as that, as soon as you reach certain voltage x ampere x time regimes the body’s resistance can start to fall, sometimes quite rapidly, so one cant really use ohms law to calculate electrical shocks.
the normal resistance before breakdown also varies quite a bit from person to person and it depends on where one measures, the papers i read on the subject measure from palm to the sole of the opposite foot.
i don’t really think we actually have a good distribution of the relative conductivity of different parts of the body.
I’ve always heard that the body is pretty conductive, once you get past the skin. People are bags of salty water, after all.
Basically, we’re all just sea cucumbers with anxiety issues…
When I measured the resistance of my body, I got a pretty much exponential I-V curve in the range between 1.3 and 15 V, with the effective large-signal resistance dropping from ≈300 kΩ to ≈12 kΩ. Then the pain became too annoying, plus I didn’t want to electrocute myself as there was nobody else in the room at that time.
But that doesn’t that story have the status of legend/myth? That article claims references without actually referencing anything that can be read.
I agree… I’ve been thinking about how this could be true. I just don’t see a small charge being able to damage the cells enough. It would stick to the blood stream and move through the aorta, avoiding the bulk of the heart. I suppose it could cause an irregular beat which could result in death assuming it interrupted the right nerves, but I doubt a 9v batter would have enough power to rupture cells. My tongue doesn’t start bleeding when I hold a 9v battery to it, and that’s an optimal situation for max discharge through the body, though very localized to the tongue.
Low voltage / current DC AND time, can kill by an electrochemical reaction. Coworker got 24 hour emergency surveillance, after touching 30KV DC. Lucky he didn’t get injured by his 4 meter jump and had no burns.
Well, since you can test a 9V battery on your tongue without killing yourself…
Dam it,
now I’ll have to go and buy new resistors,
for everything!
But the Watt Balance is intended to replace the Standard Kilogram, and that uses a measured current (as I understand it).
Yeah, I found the mention of looking for a “physical” ampere in opposition to Standards organizations moving away from “physical” standards to derived(my word for it) standards. Maybe they need a “physical” standard to be able to derive one…
Current isn’t necessarily electrons but a flow of -charge-, so a certain number of electrons flowing through a conductor does not necessarily result in a certain net current, so to actually count the electrons is missing the point.
In many, many cases, the movement of charges other than electrons is, or can be made, orders of magnitude less than the uncertainty in measurement, so only the electrons matter. In common metallic conductors (essentially pure copper or aluminum), as long as the current density isn’t too high, electrons are pretty much it.
In this case, charge carriers other than electrons, for practical purposes, are not relevant.
Still, the attempt to define an ampere in terms of electrons is similiar to attempting to define gallons per minute in terms of the number of water molecules.
It holds under certain conditions. In others, not so much. Semiconductors come to mind first.
There are no free quarks. All charges are integer multiples of the electron charge. Hence counting the electrons makes perfect sense.
In dense materials, all sorts of quasi-particles or collective excitations appear as the behaviour of the system, which can carry different amounts of charge.
There’s no reason why we couldn’t push eg. 2/3 of an electron’s charge through a conductor. It’s simply a matter of how we define the limits of the conductor and the measurement for the charge. To put it in a different analog, a person doesn’t need to be in or out of a door, they can stand on one side and bow.
https://en.wikipedia.org/wiki/Quasiparticle
“In solids, an electron quasiparticle is an electron as affected by the other forces and interactions in the solid.” … ” its mass can differ substantially from that of a normal electron;” … “Its electric field is also modified, as a result of electric field screening.”
Point being that when you observe the conductor from a distance outside of it, the screening affects how much charge is moving through the material. The sum of the electrons moving in the material isn’t the same as how much charge an outside observer feels is moving through the wire, so the “ampere” as defined by counting electrons in an ideal wire does not always result in the same effective current as defined by e.g. how much force a pair of wires would exert on one another.
An “Ampere” is 6.28 x 10 ^18 electrons past a given point in one second…. Or so I was taught way back many decades ago. What the embarassment about it is that, currently, it is based raggedly upon a physical artifact: the kilogram. (A charge between two plates lifting a weight.)
The goal is to base as many of the standard units upon something reproduceable by anybody following the same rules, but not possessing a particular artifact. In example, the second is based upon so many transitions of the cesium atom between two states of ionization. Anybody possessing the right equipment can measure it and get the same answer. You don’t need a particular batch of cesium. Right now, to properly resolve an “Amp” you would need to possess THE kilogram that resides at NIST.
Probably clears up nothing…
There are several unit redefinition efforts going on right now. I have lost track of which ones where, but there are several efforts to tie down the mole and use that as a way to tie down the kilogram. IIRC, mass is the last base unit in need of an artifact-free definition to allow all others to be defined in terms of each other and reproducible phenomena.
If an ampere can be defined or fixed independent of the kilogram, the kilogram can then be defined in terms of the fixed ampere, and the Avogadro’s constant would follow from that. It’s called the Watt balance.
It’s a competing method of defining the kilogram to the other one where they’re trying to manufacture a perfect sphere out of silicon and then count the number of atoms in it to define the mole, thereby the kilogram, thereby the ampere.
The other idea being that if you can manufacture an object of an exact known volume – a sphere to within certain size tolerances that are better than the uncertainty of mass in the standard kilogram – and you know how many atoms in a unit volume you have because you can count them with an electron microscope from a sample, you can manufacture as many mass standards you want because the kilogram can then be defined as fixed number of silicon atoms.
Can’t you just say 1 mole = 6.02214086 x10^23 atoms = 12 grams of carbon-12 ?
You can then use that to define the gram, and a kilogram is a thousand grams.
Yes you could, but then you’d subtly change all the international weights and measures insofar as the Avogadro’s constant isn’t exactly in agreement with the existing kilogram.
The idea is to count the number of atoms in a sphere of silicon exactly to get the Avogadro’s constant down fine enough for the measurement to exceed the accuracy of the prototype kilogram which is known to about 20 parts per billion.
Avogadro’s constant itself currently has an uncertainty of 12 parts per billion, and the measurement of the volume of the silicon sphere has an uncertainty of about 9 parts per billion. The combined uncertainty of not knowing exactly how big the sphere is, and how many atoms there are in a gram combine to make the accuracy of the measurement worse than what is achieved with the international kilogram prototype.
Thanks, That made a lot of sense
THE kilogram (the IPK) is at BIPM not NIST.
https://www.youtube.com/watch?v=ZMByI4s-D-Y
The Kilo is soon to be based on a number of silicon atoms. The replacement to the original kilogram weight already exists and it is beautiful.
I have a spool of infinite length and negligible cross-section wire I can sell you for a reasonable price…
But I would also need a voltage source of infinite volts to push one amp through this wire :-(
Not if it’s superconducting.
But my fridge doesn’t go down to absolute zero so the wire is useless.
I’ll buy half of it.
Cantor is getting a chuckle out of this.
I bet I can get one cheaper on AliExpress… And with LEDs on it :)
Though the “genuine” standard Kg I bought seemed to be fake.
I wouldn’t trust an administration of a country which still use imperial unit to work on standardisation… :-)
I am not sure that it will be usefull to find a new definition if it’s definied with an ambiant temperature in farenight, a presure in psi and a speed in miles per hour (strange how the imperial system don’t have its own unit for time measurment, maybe becuse its not “full decimal” with a part wich is base 10, an other 60 and an other 24…)
Are you referring to the United States of America?
If so, then you are unaware that country has officially been on the Metric Standard through an Act of Congress since 1895.
325 million people in the US are quite unaware of that. Probably a bad publicity campaign, or a lobby by the imperialist industries?
Interesting tidbit: the US of A , together with Liberia and Birma (!) are the only countries in the world still supporting the measuring system by their long forgotten imperial oppressors.
Talk about ‘independence’…
UK highways still post speed limits in miles per hour…
Yup, and most of us measure height of people in feet + inches, weight of people in pounds + stone, and weight of babies in pounds and ounces. In hospitals they write it down in metric, then convert to tell you.
Aside from that, we’re pretty fully metric.
If we converted to km for speeds, they’d manage to do the conversion at 1mi = 1km, and it’d take even longer to get to work.
Oh, and we measure land area in multiples of the size of Wales, especially if measuring rainforests.
Someone might want to notify the inhabitants, seems they missed that memo.
In an attempt to be pedantic and possibly argue semantics… We don’t use imperial unit, we use United States customary units. which are based on English units (the predecessor of imperial unit – hence why there is a US gallon and an imperial gallon).
…the Mendenhall Order (1893) saw the changing of the US standards (the actual physical objects) to metric, as well as redefining the definitions of United States customary units to based off said standards.
Someone better tell all the pilots and airlines of the world too.. (knots)
And sailors..
Not just U.S. sailors. All of em’.
Arrrrrrrr (pirate scowl)…
A nautical mile is defined as “one sixtieth of the distance between two parallels of latitude separated by one degree”… it’s got a nice relationship to the circumference of the planet and degrees of arc, which are the main measurements of navigation on/over this planet’s surface. A knot is one nautical mile per hour.
Yes they’re not SI units, but navigation is a fairly self-contained field and the nautical mile and knots are globally understood (by pilots and seafarers), so there’s not much opportunity for misunderstanding or conversion-induced errors.
Source? Only reference I find for that year is that the Utah constitution required the metric system to be taught in schools (repealed in 1987). I don’t see anything about the United States switching to metric, and I would very much like to.
I’m not sure I trust what you have to say when you don’t know how to spell Fahrenheit.
Thank you for that. I was thinking he was referring to measuring temperature over time (fortnight).
I have to admit that my spelling was as good a the measurment unit it was refering to.
By the way i was not asking to be trusted, so I gess : thank you for noticing my spelling.
Knock it all you want, imperial units are still better sized for daily life. Yes, the math works out better in metric for many things, but I’m happy using both systems where they’re each best.
In the old days, this would have been something like: 1A = the current, at 1kV, required to kill 12 adult horses connected in parallel (“12 horsepower”). Ammeters (and, by extension, power meters) were therefore expensive back then, so it was a great relief to the power industry when it was announced that nuclear power would generate electricity that was too cheap to meter.
No wonder fda approval is so expensive, finding the LD-50 of a new drug or food must require numerous mice, monkeys, and humans, and you can only reuse half of them when the excitement is done.
Lucky for me, I’m always an outlier on the bell curve.
Hmmm. If 12 horses connected in parallel give you 12 horsepower, what do you get with 12 horses connected in series?
A 3-month documentary about dressage.
gee, I hope charges in the new Ampere are going to be much faster than they used to be, because my PC is laggy as hell lately
I have a question, When electricity flows is it one electron at a time in single file?
No, sometimes they travel in packs or bunches as in a particle accelerator.
And when the juvenile electrons start hanging out in bunches at the mall, it’s all downhill from there.
It’s ok. Heat up the mall enough and they disperse very rapidly. :-P
Just don’t lose any of them while measuring.
“Darn it, I’ve lost an electron”
“Are you sure?”
“Yes, I’m positive”
“Have you lost a positron?”
“Negative.”
This statement is untrue.
Martha! Did you hear THAT?! There’s now a gummint conspiracy to change the AMP!
Why didn’t you just say that
“Proposed definition: The ampere, A, is the unit of electric current; its magnitude is set by fixing the numerical value of the elementary charge to be equal to exactly 1.60217X×10−19…” (which we have known since the famous Milliken Oil-Drop Experiment a bazillion seconds ago).
All the NIST is doing is saying is that one ampere is equivalent to one coulomb (roughly 6.241×1018 times the elementary charge) per second.
That’s all, folks. Move along; nothing to see here.
But doesn’t this mean the little internet computer in my pocket will stop working now that ohms law is officially busted!?
The physicists at NIST are freaking out, they’ll find their answer, then the guys in the actual metcal lab that actually calibrate things using this new standard will be like “well, its within a milliamp, good enough for government work”
*face-palm*
Meanwhile, children are starving and people are dying of cancer and the common fucking flu.
You obviously have never worked for the Fedeal F*****g Government: your priorities are totally messed up.
You talk as if there isn’t enough money to put bureaucratic red tape across more than one scientific frontier at a time.
Ladies and Gentlemen, the Six Trillion Dollar Budget: “We have the (red) tape. We can encumber it.”
Fixing THE Ampère: Find graveyard, dig skull and bone, find some original DNA… André-Marie wouldn’t be amused.
Why would somone possibly give a crap how many electrons there are. Even when/if you could know it exactly how would this help anything
“You can’t just order a spool of infinite length and negligible cross-section wire and have it express shipped.”
Are you sure? It’s probably in the McMaster-Carr catalog but the shipping and handling would undoubtedly be indeterminate.
You will now have to change every circuit breaker in your wiring panel.