Recharging your mobile phone or your electric vehicle in a few minutes sure sounds appealing. Supercapacitor technology has the potential to deliver that kind of performance that batteries currently can’t, and while batteries are constantly improving, the pace of development is not very fast. Just remember your old Nokia mobile with Ni-Cad batteries and several days of usage before a recharge was needed. Today we have Lithium-Ion batteries and we have to charge our phones every single day. A better energy storage option is clearly needed, and supercapacitors seem to be the only technology that is close to replace the battery.
How Supercaps Work
Batteries store energy in electrochemical form, reactions inside the cell release electrical carriers that form a usable electric current. Supercapacitors work on a very different principle, storing energy in an electric field that is created when charges of opposite sign are held separated from each other.
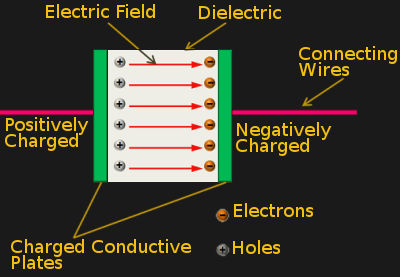
The operation of a normal capacitor is illustrated in the figure at the left. Two conductive plates are separated from each other by a dielectric medium. When voltage is applied to the plates, electrons accumulate on one plate, and are depleted from the other (forming positive holes). This separation of charges creates an electric field in the dielectric and this field is where energy is stored. Once the field reaches is maximum strength, the capacitor is fully charged. The electrons are attracted to the holes, so, if we give a path for them to flow, an electric current is established and the capacitor starts to discharge.
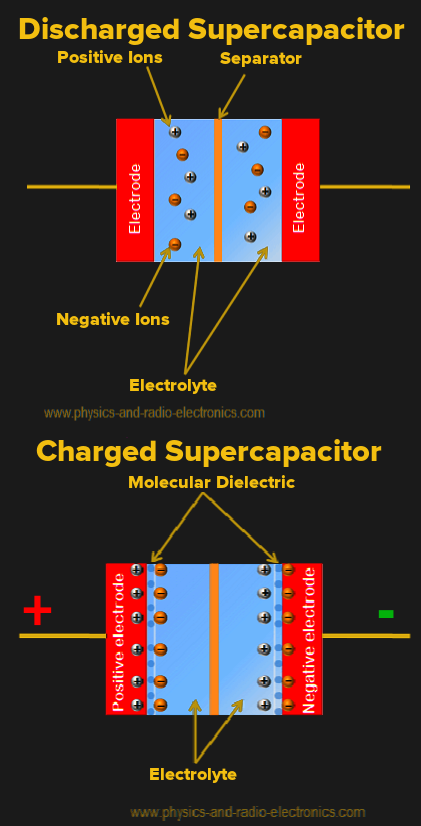
Supercapacitors have a different design, as shown in the right image. We also have two electrodes usually made of carbon, an electrolyte and a separator that allows the transfer of ions in the electrolyte. When voltage is applied to the electrodes, positive ions diffuse to the negative electrode and negative ones to the positive electrode. Electric charge accumulates at the surface of each electrode, forming a double layer (hence the name electric double-layer capacitor). Each double layer works as the simple capacitor we explained before, but we have one at each electrode. Therefore the supercapacitor is effectively two capacitors in series by design.
But why is capacitance so large in a supercapacitor compared to a normal one? The capacitance (which is proportional to the energy that can be stored) is directly proportional to the plate area and inversely proportional to the plate separation. In a normal capacitor, the plate separation is the thickness of the dielectric — on the order of tens of microns while in a supercap that distance is on the order on nanometers (one-thousandth of a micron). Also, the carbon technology used for the supercapacitor electrodes allows for much more surface area. Its spongy nature makes the effective area up to 100,000 times greater than the square area of the electrode itself.
Comparing Batteries and Supercapacitors
Right now batteries and supercaps are kind of complementary, with the strength of one being the weakness of the other. Let´s review the key parameters of supercapacitors and Li-Ion batteries:
- Charge time: Supercaps excel in this, with a charging time from 1 to 10 seconds, compared to 10 to 60 minutes to reach a full charge on a battery.
- Life: Typical batteries have 500-1000 charge-discharge cycles while supercapacitors can reach up to one million cycles. In vehicle service, batteries have a life expectancy of 5 to 10 years while supercaps can last for 10 to 15 years.
- Specific energy: This is the total stored energy per unit mass, and is the principal weakness of the supercapacitor, with an average of 10 Wh/kg, compared to 100-200 for batteries. For reference, we have 3700 Wh/kg for petrol fuel (considering 30% efficiency of an internal combustion engine). Regarding energy per unit volume, supercapacitors are also far behind at 15 Wh/L, and 1200 Wh/L for batteries. That means that a supercapacitor powered Iphone 5 will be 2 inches thick.
- Specific power: Since supercaps can charge very quickly, they can also discharge rapidly, therefore they can deliver a power of up to 10,000 W/kg. Li-Ion batteries are in the range of 2000-3000 W/kg.
- Cost: Being a relatively new technology, supercapacitors are still expensive, with a cost of around $20 per watt, while batteries are much cheaper in the range $0.5-$1 per watt.
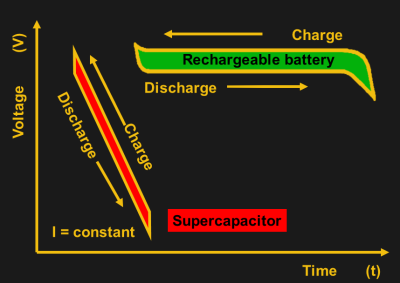
There is an additional disadvantage to supercapacitors compared to batteries: their voltage diminishes approximately linearly with stored charge, while batteries keep an approximately constant voltage until they are almost depleted. That means that additional circuitry is necessary to maintain voltage at a usable level when using supercaps, consuming some energy in the process. The typical voltage of supercapacitors is 2.5V-2.7V and, as with batteries, you can connect them in series for higher voltage. However, there are small variations in capacitance and ESR (equivalent series resistance) among individual supercapacitors, which causes uneven voltage distribution. Overvolting a supercapacitor quickly leads to failure, therefore balancing circuits are necessary to ensure that the voltage on each supercap is approximately the same.
Safety Issues
Lithium-Ion technology has had its safety issues that we all heard about, the recent incident with the Samsung Galaxy Note 7, and Boeing´s 787 Dreamliners grounded in 2013 after a battery caught fire are just two examples. Of course, given the millions of batteries out there, the actual rate of failure is incredibly low, so it is not an unsafe technology. Regarding supercapacitors, they have a much lower internal resistance than that of batteries, so in the event of a short, they do not heat as much. Sure, the technology is still in development, new materials and methods that can bring higher capacities may also increase risks, but as of today, we can say that supercapacitors are safer than Li-Ions.
When Can We Have a Supercap iPhone?
Supercapacitors already have several niche applications, with an estimated $400 million world market. Memory backup and protection was one of the first applications, as well as for powering electronic toys. They are also used in solar arrays and for micro energy harvesting systems. At the high end of the energy storage spectrum, supercaps are used in hybrid electric vehicles for regenerative braking and to provide starting power. The power grid can also benefit from them, using banks of supercaps as a buffer for power surges, the transmission lines can operate closer to a 100% capacity, increasing efficiency.
All of this is good news, and supercaps have begun to take on some roles traditionally assigned to batteries. But supercaps still lag behind batteries in terms of storage. New advances in technology, such as the use of graphene and other compounds, may increase capacity in the near future, making the supercapacitor a real option to replace the battery. For now, manufacturing remains expensive, and physical size means that even if you are willing to splurge on price you still can’t get a reasonable replacement for today’s Li-ion toting mobiles. Perhaps the next trend in smartphones will be a return to the brick design, making room for supercaps to utilize their rapid charging and long service life. Until then we wait for manufacturing advancements that can fit larger plates into a smaller space.















Not forgetting there is some interesting work being done with supercapacitor battery hybrids that have some of the features of both.
Do they explode twice?
LMAO! :)
LOL, very droll Koll.
“Will Supercapacitors Ever Replace Batteries?”
No; never.
Batteries actively generate energy; supercapacitors only passively STORE energy. Major difference.
Funny, I always thought that through chemical reaction, they released energy.
As you probably know by now. Batteries use a chemical reaction.. where as Caps. Do not.
Unless you have a nuclear battery, it doesn’t “generate” energy. It only releases energy stored in chemical bonds. So, batteries, just like capacitors, only store energy and don’t create it. It’s just being stored in a different way.
The same is true for fossil fuels. Fossil fuels are simply a store house of energy from life that existed millions of years ago and stored energy from the sun through photosynthesis. So, when you drive your gasoline burning car, your actually driving a solar powered car. It’s just a little more indirectly “solar powered”.
Well, to be fair even a phone powered by nuclear decay is charged too, thought typically requires the heart of a supernova or a breeder reactor to get full bars.
There is no organic component to the oil and gas coming from the Fuel we use with the exception of coal.
Can you explain that?
Organic:
“relating to or derived from living matter.”
What do you call the C in the CO2, inorganic?
The statement about battery active power generation is the opposite of true. I don’t even see how you could be so wrong? If you leave a battery alone on a shelf do you think it will charge itself?
The very word “battery” refers to storing, not generating, energy. Where did you get the idea that a battery is a generator?
“Battery” refers to quantity. “A battery of cells,” like a “battery of artillery.”
A lead-acid car battery contains ten cells. A 9-volt battery contains 6 cells.
A primary (alkaline, zinc-air, to pick a couple of examples) cell produces electricity through chemistry. It never gets charged. The chemistry is eventually depleted and then discarded.
These are ancient, specific, and well-understood terms.
But so generally misused that they are now equivalent terms for all intents and purposes.
At a certain point, defending the “real” definition of battery is nothing but an example of etymological fallacy. You can sit there all day and pretend “decimate” means “to reduce by one tenth”
I don’t know your special type of car, but an ordinary car battery has 12V and contains six cells with 2V each.
@HC
Thanks for this! Engineering and related fields requires a level of precision that causes some people to forget (or attracts some people who never learned) that use of language–to include connotations, denotations, grammar, and so on–can only be right or wrong insofar as there is consensus within a given group. The OEM wasn’t handed down by God, and even if it were, that doesn’t stop me from following a different god. :)
Lead acid batteries produce 2.2 volts per cell. ( 6 cells)
You cannot generate energy, at least without nuclear reactions.
Nuclear reactions do not generate energy. They convert matter into its energy equivalent. Where did the “mattergy” come from originally? Well, that’s too cosmic for me.
The first law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic systems. The law of conservation of energy states that the total energy of an isolated system is constant; energy can be transformed from one form to another, but cannot be created or destroyed.
I learned a long time ago to never say never, BUT when you look at the comparative energy available between 1 Farad charged to 4V versus 1 amp-hour at 4V it highlights the difference. 1 joule = 1 watt * 1 sec, 8 joules for a 1F cap (1/2CV^2), 14400 joules for a little 1AH 4V battery (assuming a perfectly flat discharge voltage of the battery discharging at 1C….).
Needless to say there will need to be 4 orders of magnitude breakthrough in capacity of supercap technology to equal present day Li batteries. That breakthrough (or series of them) may eventually come, but batteries are also being improved and some advances apply to both technologies. Also power consumption breakthroughs are occuring such as subthreshold logic that may reduce the requirements to the point where supercaps are “good enough” for most applications.
Just my $0.02
It will depend the application, of course, in high cycle services like regenerative braking they will likely beat out batteries, and maybe in some grid services, but never in places where long-term, on-demand performance is needed.
Agreed, good point. Considering # charge / discharge cycles over the life of the device and capability of charge /discharge rates, caps have it all over batteries, but comparing energy density, joules per unit volume, batteries will continue to win for a long time to come.
I agree, in “big” applications (off-grid storage, car starter batteries, and to a lesser extent, electric vehicles) some of the advantages of supercaps could be useful, namely their longer life and assumed lack of heat generation when dis/charging.
Will we ever see them in personal consumer goods such as phones? Probably, but only when they make them safe and economies of scale cause them to become cheaper than the current chemical batteries because who really needs a phone that can charge fully in a minute (when your charger is the limiting factor) or can dump its entire charge in a second, or last 10 years?
Absolutely right, that’s the true reason capacitors will not substitute batteries, at least not in the near future. Not the words about “generation” of energy, what is not possible in reality.
Battery does not generate energy. it just stores. like a super capacitor. it is the energy density that differs. so a super capacitor needs to be much bigger and heavier to a comparable lithium battery. Besides , the price.
Depends what battery you’re talking about.
Today I rode on Nice Tram 2 to the airport, a new and regular service across Nice, France.
Tram2 – yesterday was first public run – has no batteries, cables, or electrified lines and travels at 70kph on some stretches of track.
Power is supplied from solar power to super capacitor pads at stations that deliver enough power to the tram capacitor in 2 seconds to get to the next station.
Smooth, clean, fast. And cheap for the public at 1 euro per ride!
I’m told this is a global first.
Tram 1 in Nice – in service for 10 years or more – uses batteries that are charged via cables at stations.
Voila! Supercapacitors replace batteries!
Bugger, thought about this idea for a while, glad finally someone is doing it. At 1 euro a ride, they will made there money back quickly, as operational costs have power reduced or removed, initial cost are cheaper per km with no copper in the overhead wires or stauntons. So many good ideas around the world need to spread everywhere.
Thanks I find info on super caps interesting and useful.
I have just completed a design with supercapacitors to hold up the power supply of an industrial embedded Linux system. Supercapacitors of the double-electric-layer type can have extremely low internal resistance, such that they can provide many tens of amps when shorted. This is a safety consideration that parallels that of high-discharge Li-poly batteries.
Be careful out there.
You raise a very valid point: as energy densities get larger with both these storage devices, the potential for major harm from sudden accidental discharge increases. I’m not so sure I’d be comfortable regularly using something that not only looks like a stick of dynamite but could go off like one.
The cap can be shorted without hurting the cap. It’s what happens to the short that’s the concern.
For now perhaps, once these things start storing really significant amounts of energy how they might fail in a situation like say an impact where they were crushed will be an issue. Any rate whenever one is trafficking in large amounts of potential energy the danger goes up.
Atomic batteries. :-D
@ Ostracus – In general atomic batteries to this point have been rather low-powered devices and whatever risk that they might present would come from loss of containment and release of the radioactive isotope. But even then the quantity is so small as to be a fairly limited hazard.
Well the one’s I had in mind were of the more fictional sort. Unless there’s some amazing feats of engineering forthcoming I don’t see atomic batteries being a problem, and with glass encapsulation of the fuel leaking will not be a big issue.
I’m not a formal engineer but I’ve always enjoyed the topic.
Rather than having one big Supercapacitor, can they just set them up in parallel? To further eliminate risk, they are only connected in parallel by mechanical means, only when the automation calls for it. I understand during this process, it could still short. But at least during the time when it’s not being used, the capacitors are segmented and if one does short, just the one would be affected.
Now I have visions of a portable 12V spot welder.
Just charge the capacitors up then *BANG*.
You can do that with a large regular cap. Discharged one with a screwdriver once, and it welded to the terminals. Took some explaining to the cap’s owner…
There are lots of plans out there for capacitive discharge welders, and they’re also available commercially, aka the Sparky series of jewelry welding setups. One of the several neat things about them is they can weld stainless steel studs to gold earring backs, for instance. They’re often used for welding tabs on battery packs because they don’t heat the battery measurably during the welding process. The one I built isn’t 12V: it boosts wall voltage up to about 400V and then rectifies it to charge the cap. But you could do that with 12V just as easily.
The cap may survive, the shorting wire not
Sorry I hit the “report comment” by accident.
I have seen a wrench melt by accidentally shorting a lead-acid battery. So I do not think a supercapacitor will suffer damage in the event of a short circuit, but the tool or wire that causes it …
I’m more concerned about what might occur if a fully charged supercapacitor is physically damaged
Star Trek showed us what can happen.
And what if that is internal to the capacitor?
that’s great!
This danger applies to any sufficiently large capacitor, I recall being told about the huge capacitors, used in military radars, having to be stored with a bar across the terminals to stop them picking up a lethal charge just from the atmosphere. This would gear from 40 or 50 years ago, so long before “super” caps.
Thirty years ago we would short the terminals on our large high Voltage (PolychloroBiPhenol filled) capacitors with a small wire when they were not in circuit. Large as in approximately 7 inches thick and 2.5 x 3 feet.
Interesting. A house *powered* by the atmosphere.
Although there can be atmospheric electricity (*), these caps do not charge “from the atmosphere”. The slowly release trapped charge from the dielectricum (“dielectric absorbtion”) and that can under the right circumstances reach lethal levels.
*) Once seen: shortwave amateur radio “long wire” antenna (40m band ?) with several 10s of meters of RG213 coax and an N-connector during snowfall (very dry air) caused jumping sparks about 1/second over ~3-4mm in the connector with quite some bang and discoloration of the Teflon dielectric in the connector.
It’s not uncommon to have several hundred volts per meter electric fields in everyday conditions. It’s just that the charging current is microscopic.
Still, 10 Joules is enough to stop a heart or cause you to punch yourself in the face from the shock. If you’ve got a 1 nanoamp current going at 1000 Volts potential – suppose your capacitor is grounded at one end and a wire is sticking up in the air – it can pick up 10 Joules of charge just sitting there for three months.
@Dax:
I calculated it up to much more than 1nA:
The 50Ohm coax has 100pF/m, lets assume 20m -> 2nF
Dry air has a breakdown voltage up to 20kV/cm, lets assume a breakdown voltage at the connector of 5kV.
That gives us 10µC of charge per spark and – depending on the discharge rate (between 1/s and 1 in 10s) a current of 1µA to 10µA, what is much more than 1 nA.
Those huge caps were still microFarad.They might have been high voltage, however. As mentioned, they don’t pick up a charge as that trapped electrons migrate. If even a small percentage of the charge remains on a high voltage capacitor it can produce an uncomfortably high voltage and be an unexpected spark source, aka fire starter.
http://physics.stackexchange.com/questions/263790/main-cause-of-self-charging-of-unshorted-capacitors
and this from Bob Pease
http://www.datasheetarchive.com/files/national/htm/nsc03883.htm
When I built a capacitive discharge welder, I used a bank of 630V, 2200uF caps. The guy who gave me his stash of caps had soldered megohm resistors across the terminals because he said they got “bitey” if he didn’t. I ended up making something vaguely like a tri-state device so when the cap welder isn’t in use all the caps are shorted with similar resistors, and then either relay into ‘charging’ or ‘discharge’ states in use.
Many many moons ago I used to work in the electronics industry doing repair work. This was in the late 70’s. We got a bunch of these big boards, about 2×2 feet just plastered with TTL, and most of them had Vcc to ground shorts, but they had worked at one point in time. People clucked around with them, but it was really just shotgunning for the most part. One night I was rooting around in the stockroom looking for something and I found bunch of interesting items. One was a tub of really big compute grade caps. 5V at something like 200,000uf. These were the size of mason jars with big screw terminals on them. I also recalled having seen what I thought would be a neat paperweight. This was an SCR, but rated at hundreds of amps. One of the terminals on it was a braid that was heavier than the wire going to the starter on your car. A few nights later I had my device built. A tub of caps and a the big SCR and a pushbutton switch. You hooked the caps up to a bench power supply and slowly ramped the voltage up to about 4 and a half volts. I had a few sets of heavy wire and big alligator clips and these went to V+ and ground on the board in a few places. You disconnected the power supply, took a deep breath, and pushed the button. Most of the time the shorted device would de cap itself and it’s smoke would come out. I fixed quite a few of the shorted ones with that setup. I still have one of the SCR’s though I never did use it as a paperweight.
A great way to release the Magic Blue Smoke.
Another technology that might have some potential as a battery alternative would be a fuel cell. Although trying to fill up a cell phone sized gas tank might be messy.
Maybe not so messy. People have been filling Zippos successfully since before you were born. And modern butane ones are pretty clean and easy to fill too. There’s no particular reason a methanol refuel should be messy.
The butane refills leak like a mofo because the nozzle is often poorly fitting and the adapters are made crap. I get cold boiling liquid gas all over my hands regularily when filling various butane powered tools.
Perhaps the fill valve on a phone can be made with a little more precision than the one on a 20ct lighter.
Methanol is poisonous, so it can be a bit messy
The antidote to methanol poisoning is ethanol. Easy-peasy: Just stay drunk.
No harder than filling up a cigarette lighter.
I am no expert, but from my understanding, fuel cells release water vapor as a byproduct, and its a vapor because it produces a lot of waste heat. I’m gonna go out on a limb and say that a cellphone which operates at 672 Rankine and fills your pocket with steam wouldn’t be the most popular or useful device out there.
Fuel Cells would be useful for many other larger scale applications though.
But on the bright side your clothes are wrinkle free. Anyway the power draw may be so low that the steam generated would likewise be low.
Those flame less pocket warmers that looked like a Zippo and ran on lighter fluid were popular for a while a few years back. Just market the cellphone as a dual purpose hand warmer.
Assuming you’re human, *you* release water vapour as a byproduct, at the rate of around a gram per minute. But despite you wasting around a hundred watts of heat just sitting there supplying around 20 watts of power to your CPU (i.e. brain), it’s not coming off as steam — just as vapour dissolved in air, at a modest 37C.
You are quite correct and that is a very good point. However, from what I can find online, most (not all, but the vast majority) of commercially available fuel cells operate at or above 33 degrees Newton.
>33 Degrees Newton
>672 Rankine
You do love your obscure temperature scales, don’t you?
You mean Celcius/Centigrade?
@Thiru
I had to google it: no he does not. Newton invented a temperature scale where the freezing point of water is 0, and the boiling point is…33. I can find no explanation for why he chose the values he did for the various reference points. They’re all weird numbers.
Unless you are in an environment that is 100% humidity.
Most of that evaporation is through your breath. If you try and breathe into a scarf over your face, the fabric gets noticeably moist.
https://farm3.staticflickr.com/2190/2205713577_43ebd22e8a.jpg
Some company has developed it. It’s a sealed proprietary cartridge of course. Supposedly airline safe till hacked into a “bomb”, but only available (where and till when) from us.
This cartridges contain a hydrogen producing salt – “just add water”, I think it was called sodium meta silicide. But price ($/W) and volume/watt was higher than for normal AA batteries, the fuel cell itself not yet taken into account.
There is a little problem with that ‘charge your mobile phone in 1 minute’ though.
Lets assume you have a mobile phone that you can charge with a normal 5V/2A charger in one hour. The charger is rather small, the cable thin and the connector is micro-USB.
If you want the same charge in 1 minute, you’ll need a charger with 5V/120A. That’s a pretty beefy PSU and a hefty cable, not to mention the power connector on the phone.
Easy fix.
Make the phone body out of metal (a la iPhone) and have the top and bottom surfaces of the phone be the charging contacts. For the charger, make it a stationary dock that clamps down on the whole phone like a George Foreman grill.
Wouldn’t even need to be the whole surface I bet, you could slide it into a dock with a spring clamp and still be able to use the phone while it charges.
True, but maybe not too bad: USB 3.1 can deliver 100W, so could deliver a 25 kJ (7 Wh) charge in less than 5 minutes. If you really need faster than that maybe consider a phone with swappable batteries…
This is a valid point, but there are other things that come into play, as well.
Supercaps can endure partial cycles without harm, including total discharge, whereas batteries need complex management of the cycle to get good life.
The efficiency for charging is not 100%, and, with appropriate design, can be better for the supercap than the battery. 10Wh for the battery might equate to 8Wh for the supercap (so many open variables here, i pulled this number from thin air, knowing that the last system I worked with got about 70% of the provided energy into the Lion cells, 30% heat and controller). At 120A, the contacts would likely be surface contacts on the device exterior rather than a connector per se. Figure 25mm^2 each. The internal wires, on the other hand, would also need to be large (maybe 10mm^2 cross section) which could be an issue. The power electronics would likely be in the charging station. A 10 minute charge would be quite reasonable with smaller wires and connectors.
Likely, due to the voltage drop with use and current issues, a supercap based power supply would store and charge at up to maybe 20V, giving 1 min charging at 30A, much more reasonable, and 10min charging at 3A trivial, connector and wire-wise. This would require an internal switching supply for the hone itself, but pretty much any supercap design would.
Do I expect 1 minute charging on my cell phone? No, not int he near future, if ever, for these reasons. Do I expect supercap in the phone instead of chemical cells? At some point, maybe 5 to 20 years down the road, but I would not bet the farm on it.
Li Batteries with one cell do not need a special management, only overcharge/overdischarge protection. And partial cycles are no problem at all. On the contrary, as cell aging is highest at the extreme charging states (deep discharge or over 4,1V) they love partial cycles between e.g. 20 and 80% state of charge.
You can swap batteries to instantly “charge” your mobile phone. The same could be done with supercaps.
@John Doe
Maybe *you* can. The most popular phones these days have non-swappable batteries.
I like what it does to the shape of the phone and the design of the back, but man is it less convenient.
Don’t forget you can work ohm’s law a little to get the watts in other ways – 120V at 5A could be done with a reasonable connector. Or to fly under the safety radar – a hair less than 50V @ 12A isn’t awful…
In the US it’s 100VA and a load of >8 Amps must be shut down within 5 seconds. That’s for a power limited circuit.
If you want to charge a device with 10Wh in 1min you can not do it on a circuit with that limited power. But even in the US you have higher power mains circuits than 100W :-) So this is no natural, physical or technical limit, but just some kind of artificial law, not nature’s law. So it can be safely ignored, if you want to :-) Just don’t let yourself get caught while speed-charging your phone :-)
The battery of a cheap solar flashlight replaced with a super capacitor:
http://www.absolutelyautomation.com/articles/2016/02/19/reusing-solar-rechargeable-keychain-flashlight
looks like :
http://www.absolutelyautomation.com/sites/default/files/styles/article_image_full_node/public/field/image/160219_SOLAR_KEYCHAIN_FLASHLIGHT_SUPER_CAPACITOR_1280x720.JPG
Remember, charge = 1/2 C*V*V, where C= Farads and V= voltage. Thus charge declines in a square law manner. 1/2 the voltage = 1/4 the charge
Charge, Q, measured in Coulombs = C * V
Capacitance means the amount of charge stored per volt. It is a ratio.
In other words C = Q/V
“However, there are small variations in capacitance and ESR (equivalent series resistance) among individual supercapacitors, which causes uneven voltage distribution. Overvolting a supercapacitor quickly leads to failure, therefore balancing circuits are necessary to ensure that the voltage on each supercap is approximately the same.”
And Lithium packs should have balancing circuits for cell over/under voltage.
Matched cells in a pack makes for more usable charge.
The 2,600 Farad surplus ultra caps were in German trolley cars powered by overhead wires. The caps gave them power to run “off wire”, as in runs to close neighborhoods. Each regular stop had a set of bars overhead to enable recharge while passengers get on/off, using the very fast recharge capability. Of course, Lithium batts would run out of charge cycles in weeks, but ultracaps are rated 500,000 to 1,000,000. Yes, balancing needed for charging situation, but even more in discharge where weakest capacitors can be driven into reverse polarity. They are RATED at 600 amp discharge, but i’ve sucked out over 2 kiloamps without issues. At $7 ea. they make great adult toys. I’d say that low V hi A rail guns might be possible, otherwise . . . . .?
6 of my 7 are going to replace failed lead-acid gel cells in my car start jump box. A 2P3S arrangement will charge quickly off of any functional car, and be switched to 6S (15 volts, to jump dead car). Since you have 14.4-14.6 volts in a charging L-A battery, cars can withstand 15V very easily.
There is another technology that works a bit like caps but does not require any nasty chemicals to be made and has an infinite recharge and discharge cycle. Compressed air. There are a few implementations that have shown it is viable. And with a well constructed container the venting in the event of an accident can be controlled (well placed week spots) so that it is not dangerous.
But seems we’re all still content in saving the world by digging more crap out of the ground and polluting 3rd world countries at the same time.
We’re a lost cause. Just glade i’ll not be around when global warming has caused mass crop failures and starvation. It’s going to happen…….!
Sweet dreams.
Unfortunately the Ideal Gas Law has something to say about the efficiency of such storage, and the thermal losses are so great that this is really not a viable way to save energy.
“their voltage diminishes approximately linearly”
That is so wrong, dead wrong. Even I ain’t much math-oriented guy but damn the discharge curve is typically exponential and nowhere near linear.
example: if discharge time of a 10Volt cap is 500ms, after 100ms it’s 3.7 volts (63% less voltage in 20% of the discharge time)
google capacitor discharge graph and tell me it’s linear. No just no.
You’re thinking of an RC circuit, where the load resistance is constant, but current draw decreases as the voltage drops.
The graph is referring to a constant-current discharge.
In real applications, we often have constant POWER circuitry, so the real performance is different again!
For an RC circuit that’s true.
However, for a constant current load it’s a linear drop:
iC = C * dV / dT.
iC = constant current into or out of a capacitor.
C = capacitance in Farads.
dV = amount of voltage change
dT = amount of time change.
So for a constant current draw of 1 Amp, in a 1 Farad capacitor, the voltage will decline by 1 Volt per second.
1A = 1F * 1V/1Sec.
Charge is not = 1/2 C *V^2, rather it’s Ec or Energy stored in the Capactor.
Energy, measured in Joules (watts seconds) stored in a capacitor = 1/2 * C * V^2.
Thus if you know you need a specific amount of energy to be stored (Joules, or Watts * Seconds) and you know the available capacitance or voltage, you can determine the latter that you need to have enough energy to perform the work that you want done.
Not all of the energy stored in the cap will be available to use since your circuit load likely won’t operate at really low voltages, so you need to compute the minimum operating voltage for your circuit and subtract out the energy that you can’t use from the total.
Perhaps with ultra-thin dielectrics, some super nano material, we will see higher density supercaps.
I’d love to see this in wearables, battery life will always be a problem with small devices. Maybe have a quick charge device for smart watches that functions as a backup to the battery.
I can see a merging of low level beta radioactive emission and the barrier in the double layer cap, Atomic battery! Slow charging for years, with registered disposal. It would have to be sized for heavy use expected.
Super caps have replaced batteries in many designs of mine and of many people, but they will never replace batteries proper
For everything supercapacitors look up robert murray smith on youtube, the guy is unbelievable!
Meanwhile, on the opposite end…
The EFL700A39 is a really interesting battery…
10$ for 0,7mAh? You have a different idea of interesting things than I. I would call it a curiosity. :-)
That battery fits in a credit card.
It’s paper thin.
Stack em.
Its rechargable.
It’s a first generation of that technology, and you don’t find it interesting?
It is paper thin – yes. So it may have some niche uses. Stack em? – No. Too expensive. :-)
OK, it is interesting from some point of view, but way to expensive for most applications.
Are super caps better than Flux capacitors that can store 1.21GW of power?
Given their current issues, supercapacitors might be best in home/solar installations. There, their weight and size would matter little and their longer lifespan would be a major plus. And the bulky gear to deal with their voltage swings could be incorporated into the existing gear that converts stored power to useful power.
Chemical storage batteries are still far better density wise.
I live in the north east. I’ve always imagined storing solar energy as heat, then using the stored heat, to heat my house (hot water base-board heating.)
That seemed like a logical way to approach it.
My electric bill and usage aren’t that high.
Better in that heat storage itself is very useful for example laundry, bathing, and even cooking/baking.
For laundry you would need a special washing machine with separate hot water inlet. And for 90°/95° wash it will still need an electric heater built in. Cooking/baking with hot water as energy source is also quite difficult.
@ Annie – No you can get some models that heat the water internally and thus have only the single water inlet. This is the norm apparently in most of Europe, and the U.K. at least. Same with dishwashers.
“Just remember your old Nokia mobile with Ni-Cad batteries and several days of usage before a recharge was needed. Today we have Lithium-Ion batteries and we have to charge our phones every single day.”
That’s a little unfair – our phones today are an order of magnitude more powerful than a desktop computer back when I had that Nokia.
Manufacturers have left utility behind in the quest for more (sometimes useful, sometimes not) features to wow the consumer. My shitty old phone with no frills lasts a week with the crapped out Li cell that is half the size of a spanking new phone that lasts a day!
Virtually any graph of energy density comparing NiCd to LiIon shows LiIon is *significantly* better.
It is not the fault of the NiCd batteries, it is the fault of the consumer!
I think you’re both going senile, assuming you actually remember nicad powered phones. My Nokia 100 from the nicad era barely got through the day on standby and talk time of an hour was considered good. A desk charger with spare battery was essential. It wasn’t until first low voltage then lithium batteries that we got the week plus standby times. Early 2000s.
So true.
I just tried to remember, my first phone (ericsson 337, long before it was ‘sony-ericsson’) had a NiCd or NiMH and I had to charge it about as often as my Galaxy S5 today, every 2-3 days. But it had only a tiny green/black LCD and mobile internet was not yet invented. It was 1997 and internet was via landline modem with 33,6kbit/s.
Later on the Nokias with LiIon batteries made a week of standby time and several hours talk-time possible.
I’ve been lurking this site for years now, never commented before, but since it’s almost a tradition, i’ll go ahead and post the obligatory, jaded post that plagues every interesting tidbit of a post on this site.
Not a hack
Would a super cap powered phone need the same battery life as a Lipo one? Charging is only a pain because it’s slow. If it charges in 10s, recharging it more regularly isn’t such a pain. People used to bitch about having to charge every day instead of every week…
Q: “Will Supercapacitors Ever Replace Batteries?”
Supercaps were invented in the 1950s.
NEC and Panasonic sold supercaps as consumer products in the 1970s.
For all you millenial airheads, the date is 2017.
A: No.
https://www.wired.com/2011/06/tiny-rotary-engines-could-power-gadgets-with-gasoline/
shows an idea from a while ago – make a tiny generator that will fit inside a wearable.
SuperCapacitors.
May I please remind?
You have a device with phenomenal coulombs plus astonishing discharge rate and a wave-front traveling near the speed of light.
Some such as the CO2 Laser I was once tinkering with require including the wavefront speed of light in the formulae. Did you calculate how thick your rubber gloves need to be? The capacitor was a 12″ x 12″ epoxy glass double sided copper clad board.
Hacking still includes calculating…. if you intend to survive.
And I advocate hacking! But DO THE MATH when it gets to high energy.
If you have…. then please hack on! Then impress us with your report.
Obits I will find on my own.
One of the manufacturer of Supercapacitors http://www.nordmate.com
Can’t wait until they put a cape on them and a big SC on its chest and put them in phones, tablets, laptops, cars, trucks (go Rivian… Normal, Il.).