I take coffee very seriously. It’s probably the most important meal of the day, and apparently the largest overall dietary source of antioxidants in the United States of America. Regardless of whether you believe antioxidants have a health effect (I’m skeptical), that’s interesting!
Unfortunately, industrially roasted and ground coffee is sometimes adulterated with a variety of unwanted ‘other stuff’: corn, soybeans, wheat husks, etc. Across Southeast Asia, there’s a lot of concern over food adulteration and safety in general, as the cost-driven nature of the market pushes a minority of vendors to dishonest business practices. Here in Vietnam, one of the specific rumors is that coffee from street vendors is not actually coffee, but unsafe chemical flavoring agents mixed with corn silk, roasted coconut husks, and soy. Local news reported that 30% of street coffee doesn’t even contain caffeine.
While I’ve heard some pretty fanciful tales told at street side coffee shops, some of them turned out to be based on some grain (bean?) of truth, and local news has certainly featured it often enough. Then again, I’ve been buying coffee at the same friendly street vendors for years, and take some offense at unfounded accusations directed at them.
This sounds like a job for science, but what can we use to quantify the purity of many coffee samples without spending a fortune? As usual, the solution to the problem (pun intended) was already in the room:
Povidone iodine (also called iodopovidone) is an antiseptic listed on the WHO List of Essential Medicines. It is composed of a water-soluble polymer called povidone, hydrogen iodide, and elemental iodine. The elemental iodine forms a complex with povidone that slowly releases elemental iodine that dissolves into the solution that already contains hydrogen iodide.
Chemistry in the Kitchen
Elemental iodine is a fairly reactive substance. In our case, it forms triiodide ions (I3-) in the solution, giving it a dark brown color (yellow in dilute solution) and acting as an oxidizing agent. It also has the very useful property of forming a starch-triiodide complex that is an incredibly dark blue. This lets us use soluble starch as an indicator for the presence of triiodide ions in a chemical assay called an iodometric titration.
Various foods contain a mix of soluble and insoluble starch – in my case I used tapioca starch and it worked reasonably well as an indicator, and it’s commonly available in nearly every local household.

We can now detect triiodide ions in solution very well, but where does that get us? Consider the composition of dry coffee:
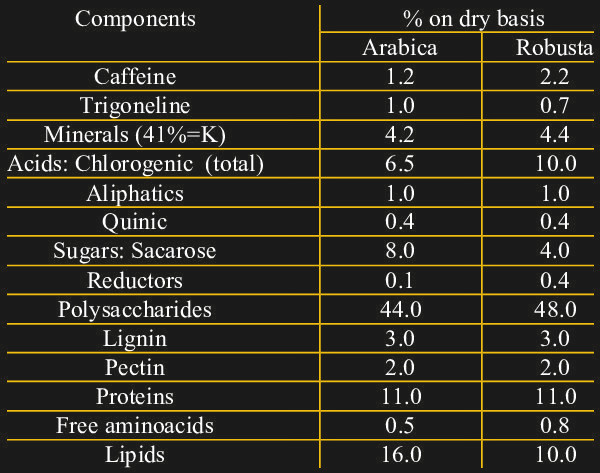
Note the relatively high concentrations of both caffeine and chlorogenic acids. While caffeine is probably familiar to most of us, chlorogenic acid wasn’t at all part of my daily vocabulary. It turns out that these compounds are responsible for much of the astringent and bitter taste of coffee, and that they are produced in higher quantities when the plant is under stress. Robusta coffee, as is commonly grown in Vietnam, also has more of these compounds as opposed to the more expensive Arabica variety. Note that chlorogenic acids do not contain or generate chlorine, it’s just an etymological coincidence.
Some people might call these chemicals ‘antioxidants’, but for our purposes they are something related but more specific: reducing agents. Reducing agents are substances that have a tendency to react with oxidizing agents by losing electrons to them. As such, we expect the caffeine and chlorogenic acids to react with the iodine this way. In theory you can make a terrible electrochemical cell with this (let us know how it goes).
In summary, caffeine and chlorogenic acid are major components of coffee. Iodometric titration is a known analytical method to determine the concentrations of both compounds. A combination of tapioca starch and povidone iodine is reasonable candidate for a reagent to perform an approximate titration. Overall it sounds like a reasonable test for the amount of coffee in something, if we create a standard reference solution.
First Reference Tests
As a side note, one study found green coffee beans from Vietnam to have the highest antioxidant load of any coffee they characterized. This bodes well for our test, as it might create a bigger local differential between genuine and fake coffee.
I arbitrarily mixed up a batch and brewed some coffee, then slowly dripped coffee into the mixture while stirring. It appeared to work!

The next step was to prepare standard solutions of coffee and reagent. After some tests, I found that a 1:200 mixture of 10% povidone iodine and tap water mixed with 5 grams of tapioca starch worked reasonably well. For the standard coffee solution, I brewed 20 grams of Trung Nguyên ‘S’ (a common local brand) ground coffee using a traditional ca phe phin and 150 ml boiling water, then diluted the result 30:1 to give about 300 ml of standard coffee solution.
The reason for the 30:1 dilution is twofold. First, Vietnamese street coffee is sold as a thick syrup that is poured over ice when served; it’s way too concentrated relative to our reagent so better to dilute it. Secondly, I suspect the reagent is sensitive to light, so it’s practical to mix lower concentrations of reagent fresh for every testing session.
As it turned out, it took 2.0 ml of the standard coffee solution to completely change the color of the soluble starch in 10 ml of reagent. Armed with that knowledge, it’s time to design an experiment, get on my motorbike, and purchase a frightening quantity of street coffee.
A Coffee Run for Science
Stay tuned – the samples take a while to process, but we’ll have results in a few days time, along with a full description of the research design. I’ll also be visiting what locals call “The Market of Death” to try to source the alleged fake coffee… I’ll try not to get stabbed and dissolved in acid.
In reality, the market is a pretty neat place and I’m often in the area. Chemical safety issues aside, it’s a really exciting place if you love chemistry and aren’t prone to nightmares.
As an aside, there’s no reason this method couldn’t be used to characterize other substances, such as ascorbic acid (Vitamin C). I tried this as well using over-the-counter tablets and they reacted readily, but decided that investigating a longstanding coffee adulteration scandal was more exciting than doing a study on fruit juice concentrations.
It may also be possible to use the solution to detect the illegal addition of sodium metabisulphite to pork, in order to sell it as beef at a higher margin. At a cost of USD 0.002 per test, we might be onto something, and further investigation is required to see if a similar method could be applied practically.
















Is there not a need to control for false positives? I would think that in selecting fillers and non-coffee chemicals one would need a similar amount of acids to approximate the taste. Do those fillers and replacements not have similar iodometric titration to the chlorogenic acids you are testing for?
^This^
For substitutes like chicory and dandelion root, the works been done. https://www.sciencedirect.com/science/article/pii/0308814687900422
The exact numbers are behind a paywall but suffice to say, they’re much much lower so they shouldn’t impact the tests.
Compounding the problem, if you just cut the coffee with filler, you’ll still get a similar enough flavor profile, they may fail a side by side taste test with real coffee but for most purposes people won’t notice, or if they do, chalk it up to watered down coffee.
Chicory is mixed with Coffee as a standard practice in India. Its very rare that you get 100% coffee in India.
Yes there is a need to test for that! I’ll be purchasing the artificial flavoring chemicals allegedly used in street coffee to make sure that they do not react as readily with the test mixture (stay tuned). Also by measuring each sample multiple times I’m roughly working out the test precision. So far it’s acceptable for the current purpose, but not mind-blowing, so there is certainly room for improvement.
In terms of accuracy, it can definitely tell the difference between coffee samples and this is repeatable. In other words if it measures two samples as quite different once, it has always done so when I ran the test again.
Overall there are a lot of things that could fool this test (e.g. ascorbic acid). On the other hand, unless street coffee vendors are specifically trying to fool it, it seems unlikely that whatever adulterants in the mixture will react exactly the same way as actual coffee. I don’t really know yet though — this is all rather experimental. We’ll all know more once I finish processing the results.
Slight spoiler: Preliminary results are that adulterants (or at least what I had on hand) do react with the test mixture, but much less, as Leithoa suggests below.
Cheap GC-MS is a thing that exists. The cost is mostly the meatbag running the gear, so it ought to be dirt cheap. You’ll want a known-good control. You might even identify where the beans were grown from isotopic distribution. That would, again, require very trusted controls.
Not a hack! :-)
Cheap if you live in the West perhaps, how common analytical labs willing to deal with individuals in Vietnam are, I don’t know but I suspect, not very.
I look forward to seeing your results! One thing: You say “Green coffee beans from Vietnam to have the highest antioxidant load of any coffee they characterized. This bodes well for our test, as it might create a bigger local differential between genuine and fake coffee.” Might this test also show a difference between real coffee from Vietnam and real coffee from somewhere else? I suggest you try some real coffee from another country as an additional control, otherwise you might conclude that the Vietnamese stuff isn’t coffee when in fact it’s real but low-antioxidant imported coffee from somewhere else.
Yes, it’s very likely but for a less exciting reason!
Vietnam mostly grows Robusta variety coffee. Arabica variety is considered higher quality overall, but has a lower caffeine and overall antioxidant load.
So you would quite possibly be measuring the difference between two coffee varieties rather than a regional difference.
If you control for that, I suspect the accuracy of the test could be improved to the point where you would be able to tell the difference, but it would almost certainly require more than the current setup. Proper starch solution (not tapioca), actual iodine solution (not adsorbed in povidone), and a burette pipette might be enough. I’ve also read that other chemicals are added to the test mixture in analytical iodometry, e.g. sodium thiosulphate.
In other words, there’s a lot of room for improvement if you have access to reagents and tools. I guess my goal here was to develop a “good enough” test anyone could perform with commonly available materials.
Supposedly the thing for making fake coffee in the United States is roasted acorns. Might be worth brewing a batch of “coffee” out of acorns to see what results you get with the test.
That and roasted ground chicory root.
During the Depression, some people drank a roasted barley beverage as a substitute for coffee.
I think Ralston Purina had a brand, as did Post Cereals.
http://jeffwerner.ca/2004/12/postum_coffee_s.html
And they had a very bizarre advertising campaign for it: http://www.lileks.com/institute/comicsins/comics/coffeenerves/index.html
I remember grandma brewing acorns in USSR in the 80s. Can’t remember the taste, I guess it was ok but not very close to coffee.
Have you done the stoichiometric calculation to determine whether the ’10 ml of reagent per 2 ml of coffee’ result produces a reasonable value for expected percentage of reducing agents? Or tested against a standard chlorogenic acid solution?
I’d love to do that, but don’t have access to those things! This is the main reason I made the test relative to a ‘standard coffee solution’. I did test different concentrations of coffee to make sure that less volume was required in the titration, e.g. that just adding a little coffee and mixing long enough wasn’t sufficient to change the color.
I suppose I could use vitamin C tablets (ascorbic acid) to work ‘something’ out, but I’m not sure how I would apply it to the current test. I know it reacts readily in both iodometric and acid-base titrations, so that would let me learn a little.
Anyway, I would love to see someone independently verify my test mixture. If they prove my test useless, I’m happy to shake their hand and thank them for their work, because science.
It has been a problem for centuries, because the one thing that hasn’t changed in all that time is human nature.
https://archive.org/stream/b21957411#page/236/mode/2up/search/adulterations+of+tea
That book is a fascinating read and that chapter on various test is very interesting. If you are wondering how the hell I remembered some obscure fact out of an old book from 196 years ago well that is simple, by coincidence I was reading that page yesterday.
Interesting, thanks!
Hey where did RW’s STFU comment go? You horrid little prudes that was hilarious.
Hell if I know what’s in it, but please someone send a truck load to my Twitter enabled POTUS, and other similarly enabled US politicians.
You should probably try to get your hands on elemental (or at least tincture of) iodine since povidone is going to have it’s own dissociation rate that will be influenced by the various pHs of your coffee/starch solutions or other unique traits to a specific vendor. And get some distilled or DI water if at all possible, loads of things in tap water could end up interfering with your titration.
It sounds like you’re doing the experiment backwards.
The write up sounds like you’re adding coffee to end-point iodine/starch solution. What you should be doing is adding excess iodine to the coffee with a starch indicator and titrating a standard oxidizer (typically a thiosulfate, another common chemical) into it. You will get a reaction adding coffee to the indicator solution but you’re opening yourself up to errors. It’s a much better practice to add standard solutions to unknowns.
Standard solutions are only standard if you know the concentrations of them. a 30:1 dilution of an unknown sample is in no way standard. Sure you know exactly how much ‘coffee’ went into it but you have no idea what the concentration of the coffee, caffeine, or chlorogenic acids are. Measuring the coffee grounds weight helps but not terribly, since steeping, grind coarseness and half a dozen other things affect extraction.
Well given that the coffee was made with the local water, I don’t think distilled would make much difference in the testing solution.
But it does affect the concentration of the iodine solution. A sample run at the start of your work may be quite different than one run at the end. If DI / RO water can’t be found then a multiple standard and blank sample should be run. It’s a good practice anyways but definitely required when your iodine solution may be degrading while you work.
Some of the iodine will also be consumed by the free chlorine in the tap water (you can use iodometry here too, so it’ll affect your blanks), though depending on the QC at the nearest water treatment that may not be likely (quick google says most cities don’t meet their standards)
You’re correct, most iodometric titrations are done with thiosulphate. I could probably source some here, but I wanted a test that could be run without depending on hard to source materials. I expect that for this particular experiment high accuracy is not necessary, so I decided to try it out with the most basic materials possible and see if I could get meaningful results. I’m certainly not trying to measure absolute concentrations of any substance — relative concentrations is sufficient right now.
I’m not sure there really is a ‘backwards’ for titrations — you can drip an unknown sample into a mixture of a known concentration and an indicator, or the reverse. As long as you know the volume used, you have a useful measurement. You’re correct again though — most labs add a reducing agent and add known iodine until it turns blue. This might be more practical!
I’d like to say that I did it my way by design, but basically I flipped a coin mentally. If my test works at all, it should work just as well (or better) adding known iodine to a mixture of coffee and starch. If I run the test again, I’ll give it a try!
I think you’re right (a third time!) about calling it a ‘standard coffee solution’ — it really isn’t. I should have said ‘reference coffee solution’. It’s purpose is just to give some context, e.g. what does it mean when it takes 6ml of coffee to titrate the test mixture? Is that more or less ‘pure coffee’ than something we might brew at home?
Anyway, all good suggestions and I’ll certainly refer to them if I ever take this test out of the experimental stage! Thanks for your time!
>>I’m not sure there really is a ‘backwards’ for titrations
From a chemical standpoint there isn’t, it’s just a best practice (and often easier) to add your solutions of known concentration to the unknown. If you end up using the back-titration method it’s required since you’ll need to consume all of the excess iodine in order to get useable numbers and adding more unknown won’t help.
>>I should have said ‘reference coffee solution’
Reference solutions are also solutions of known concentration, typically reserved for very precisely known concentrations like those acquired from big labs with insane QC measures. The type of things NIST and other metrology organizations sell. Diluting an unknown solution doesn’t really have a specific name that I know of. There are half a dozen names for solutions of known concentration but the most you’ll get for an unknown is ‘analyte’. Kind of frustrating.
As for sodium thiosulfate, it’s used a lot in pools / pool maintenance and black and white photo development. I don’t know how tightly chemicals are regulated in Vietnam though, so that may not help.
Pet stores sell sodium thiosulfate for neutralizing chlorine in tap water for aquarium fish.
Chemical regulations here are quite reasonable. You can purchase most things that aren’t outright ridiculous, and ‘chemical supplier’ is more likely to be a brick-and-mortar family business than a huge supplier, so you can just go there. I’ve also heard of nice places to buy lab grade glassware near some of the technical universities, but haven’t been able to find them yet.
Thanks for the tip about the thiosulphate! I might be able to find it through that route. I think it would vastly improve accuracy and precision without being too hard for your average hacker to source. If I do a follow up I’ll be sure to give due credit.
If you’re looking for adulterants in an organic solution, wouldn’t absorption spectroscopy be a quicker “yes/no” Test?
How about you just don’t drink drugs every morning instead? May save you a bit of time and hassle.
Drugs are good, mmmkay….
http://www.cnn.com/2017/07/10/health/coffee-leads-to-longer-life-studies-reaffirm/index.html
Most of the things I do are a hassle. Some of them very much so.
When the struggle is against my own ignorance, at no point is the universe more beautiful or my mind more free.
Some people laugh at me. I do too! If I can’t do that, what’s the point?
well put, sir!
“…don’t drink drugs every morning…”
I wonder what can be drunk then?
Tea has “drugs” as well and most of all other infusions have there own little “drugs” (which is why people have been drinking them for yonks!).
Milk is full of antibiotics and what else.
Tap water is full of chemicals.
Rain water is boring. And acid rain tastes like … acid?
I know now: we should drink B E E R !!!!!!
:-)
You’re in Vietnam, should be able to get top-notch beans! The pea-berry ones are excellent.
I’m a local in VN, excited to see your results :)
As a non coffee drinker I wonder if it matters if it’s some roasted bean or another roasted vegetable. I mean if you can’t tell by taste.
I don’t think coffee is some health product, and that you are deprived of something you need if it’s not real.
It’s different if you start to roast much more unhealthy crap as a replacement of course, but that hasn’t been established either.
“part of my daily vocabulary. It turns out that these compounds are responsible for much of the astringent and bitter taste of coffee, ”
Has anybody ever noticed astringency in their coffee?
I have never noticed it myself :(
I have been trying for about a year now to understand the unexpectedly high levels of sodium in many of the coffees which Trung Nguyen (A major V.N. coffee exporter) is selling in the US. I had not realized that adulteration might be such a pervasive problem. You can add sodium bicarbonate, citrate, and (probably) glutamate to the list of possible additives.
What better excuse for a Wawa run? More Coffee.