A basic scientific tool for chemistry and biology is a colorimeter device used to measure which wavelengths of light a particular sample solution absorbs. Some applications of colorimeters are measuring pH or chlorine levels, measuring pollutants, such as oil or pesticides, and, in some cases, can even be used to measure RNA/DNA concentrations. Even most washing machines today have a specialized colorimeter sensor, of sorts, to measure turbidity (cloudiness) to provide feedback on the cleaning process. To help in building your home scientific lab, [IORodeo] has released an Open Colorimeter.
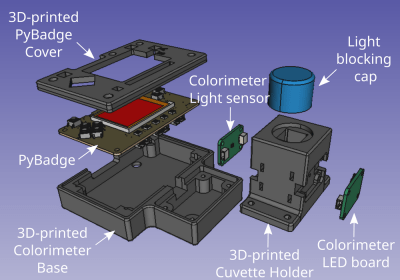
The Open Colorimeter is a self-contained device that accepts cuvettes filled with liquids for testing. The basic structure is an LED mounted onto a board that shines through the cuvette filled with a sample that is then measured at the other end by a TSL2591 color sensor. The Open Colorimeter has separate specialized LED boards for a range of wavelengths from 470nm to 630nm and incorporates a PyBadge that serves as the main microcontroller, as well as display and input.
[IORodeo] has done extensive documentation on the assembly, usage, and testing of the device. They have also provided protocols for the measurement of Ammonia, Nitrate, Nitrite, and Phosphates in addition to providing resources for absorption profiles of many other substances. All files relating to the 3D enclosure, firmware source code, schematics and Gerbers are provided under an open source hardware compatible license. For those not wanting to build it themselves, [IORodeo] is offering them for sale.
This isn’t the first time we’ve featured colorimeters, with some building a DIY version and others using it in a Tricorder project. The Open Colorimeter is a nice addition to this list and is ready for hacking and extending!