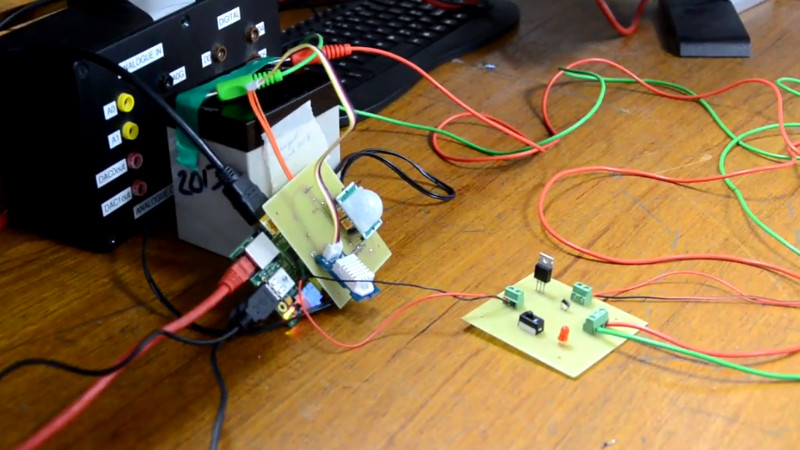
[Revanth Kailashnath] writes in to tell us about an interesting project he and his team have been working on for their “Real Time Embedded Programming” class at the University of Glasgow. Intended to combat the harsh and dangerous winters in Glasgow, their system uses a Raspberry Pi and a suite of sensors to automatically deploy a brine solution to streets and sidewalks. While the project is still only a proof of concept and hasn’t been deployed, the work the team has done so far runs the gamut from developing their own PCBs to creating a web-based user interface.
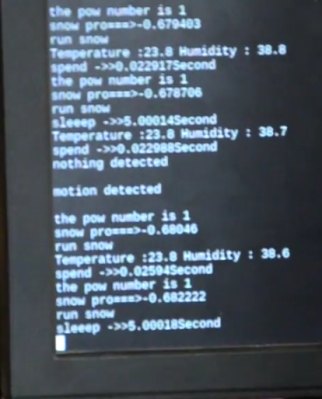 The core idea is simple. If the conditions are right for ice to form, spray salt water. Using salt water is a cheap and safe way of clearing and preventing ice as it simply drops the temperature at which water freezes. The end result is that the ice won’t form until it gets down to 10F (-12C) or so. Not a perfect solution, but it can definitely help. Of course, you don’t want to spray people with salt water as they pass by, so there’s a bit more to it than that.
The core idea is simple. If the conditions are right for ice to form, spray salt water. Using salt water is a cheap and safe way of clearing and preventing ice as it simply drops the temperature at which water freezes. The end result is that the ice won’t form until it gets down to 10F (-12C) or so. Not a perfect solution, but it can definitely help. Of course, you don’t want to spray people with salt water as they pass by, so there’s a bit more to it than that.
Using the venerable DHT22 sensor the team can get the current temperature and humidity, which allows them to determine when it’s time to start spraying. But to prevent any wet and angry pedestrians, a HC-SR501 PIR motion sensor is used. If the system sees motion it will stop for a while to let the activity quiet down.
Monitoring the sensors and controlling the pump is done by a daemon written in C++, which also logs data to an SQL database, which in turn feeds their PHP web interface. In the video after the break, [Revanth] demonstrates how the system is constantly making decisions based on the input of the various sensors. Environmental data and motion is analysed every few seconds to provide a real-time solution.
We’ve covered a few projects aimed at melting ice and snow by heating concrete, but it’s interesting to see a “smart” approach to this common winter annoyance.















That is useless when there snows that much. The salt will just make the snow mush. How would you even spray the salt? I say useless. Use sand or grit.
Thousands of jurisdictions around the world have experienced otherwise, saving much damage and lives.
Brine or other solutions are often sprayed in liquid form onto ice or roads expected to form ice, to prevent it forming in the first place. For example, bridges (even saw this in Arizona), where overnight freezing air moving under the bridge means the road surface will freeze, whereas on the road-on-ground leading to it won’t get cold enough. And after snow clearing where I live in Canada, so it minimizes the formation of or melts the ice formed by driving on the snow (which is, well, fluffy ice). We also end up with a tiny to small residual level of that treatment lying dry on the road waiting for the next snow or freezing rain. In other parts of Canada, and the world, it is too cold and is useless, except in early or late winter, which may be too small of a useful window in some areas. The temperature determines what is put down.
A recent example: two mornings ago, the lee side of my car was bare, but the windward side had freezing rain on it. Around 3/8″ thick near the bottom (partially shielded by the car next to it), 3/4″ to 7/8″ at glass height, and barely shy of an inch at the top corner to the roof, and 1/2″ across the roof and windshield. A hard strike at the top corner will get that breaking off in chunks up to 6″x2′, and it comes of in chunks or sheets too for the window once you’ve chipped down to the glass for a 6″ or 8″ trough. But you have to watch for the chunks falling on you and then trying to walk on them as you move around the vehicle to clear it.
That freezing rain fell on the fields, parking lots, houses, hydro lines and ROADS too. Fortunately they’d sprayed the roads. Can’t spray the hydro lines. Many parts of the city without power. Heard fire trucks scrambled nearly every hour. But this is so common, I only heard a few mention the PITA of getting the ice off, and no comments about how thick it was – not thick enough to be worth mentioning. I did get a casual thank you from a neighbour whose car I’d cleared the ice off of, as she needed to get to a daily cancer treatment (casual, as people just do for others who can’t – normal here). (it’s actually hard to get off when it’s thinner and the temperature drops more – some will spend an hour or hours getting it off) A few text/emails flew around among friends about who did, didn’t, or got-back power. Par for the course here, two, three times a year.
But with the spraying of solution, we don’t have to worry about the roads. Unless they get surprised (changed forecast) and don’t get it down before it falls and you have to head out then, but then there’s warnings on the news. Miserable if you’re taking the ice off while the freezing rain is still coming down.
The snow is gone already here, but if that falls onto snow and temperatures drop below freezing before there’s rain to ruin the ice, the snow cushions the load and we get the kids heading out skating on the ice on top of the snow, across the fields for miles. Only happens a few times a decade. Or some will play hockey on the ice on a parking lot if there’s not been salt nor solution put down. You can get a load of kids yelling “Noooooo!” at someone coming by with the salt.
For roads salt is good, but for pedestrian walkways it’s useless.
It works well for roads because the cars “grind” the ice into the salt and keep things mixed. On pedestrian walkways the bit of traffic that is there isn’t enough to stop the salt from just flushing away or mix it with the snow/ice
I’ve used salt on low traffic foot-only sidewalks and had it work just fine. My only objections are price and what it does to the grass.
Bull.
I’ve been using it for decades on our driveway and walkway.
Same for our sidewalks, entrance paths and walking paths.
Firstly: as soon as the salt is put down it provides grip when you walk on it. Instantly due to its physical grip, and with very quick improvement in under ten seconds, typically under three seconds, as it starts its melting. One learns to walk a little bit slowly when spreading salt ahead of yourself by hand, so you don’t end up on your butt.
Secondly: remember that snow is crystalline ice structures. Think of it as Fluffy Ice. In other words, salt melts snow too.
Thirdly: as soon as the salt is put down, it starts melting the ice/snow it is in contact with. That becomes a salt solution, which is in contact with ice and melts that ice, so as the area presented with the salt pool increases, it is melting outward from each piece of salt where it was put down. It tends to work outwards more on ice, and more downwards on packed snow (if the pieces of salt aren’t fine enough to “cover” the pack, then you end up with a collection of holes in the packed snow, which takes longer to spread and melt it away; I’ve had to dig to the bottom of a bag/bin to get the crushed/broken salt for heavy packed snow for faster results; the coarser pieces stay in place longer as they take longer to melt, so are great for treatment when it’s snowing).
Fourthly: when snow falls on a salted/solution surface (leftover from the last salt treatment, or put down in advance based on forecast), it starts melting.
We tend to keep containers of salt around pedestrian walkways where they are needed.
For my elderly father: a bag beside the garage, just outside the front door, in a bucket just inside the front door and a bag in the van. A handful (with gloves) will do the walkway for eight to ten feet. Two to three slings of a handful will do the driveway. Part of a handful instantly makes getting in or out of the van safe for him, at home, shopping or doctor’s appointment. Cheap and instant to throw a handful down for the old lady beside you.
Where I worked where we had a daycare on the ground floor, with young moms coming and going with children in strollers, walking or being carried, we kept a large bin of salt beside the front door for the whole season, with a scoop in the bin for spreading, allowing anyone to spread it as needed. On ice and packed snow, I could do the entire 60′ entrance lane, its sidewalk and their break-area with three to four aggressive flings of salt. Where I’d had to carefully ice-walk with a lowered COG up the slight slope to get to the entrance when I’d arrive early in the morning (or if too icy, abandon that for the snow covered grass to get up the slope), I could fling the salt back down the slope and immediately walk on it with confidence. Then I’d take another scoop and walk it again to spot check the surface for spots that didn’t get enough salt, so the moms could immediately walk there while holding infants without any fear of slipping/falling. Moms had to be kept safe, doubly so as they’re the primary caregiver of their child. And as some of our moms couldn’t afford a stroller, and as a mom falling on an infant could injure, cripple or kill them, this wasn’t an optional treatment of the snow/ice surface.
The sand is of very limited benefit on our ice/snow. New fallen snow sits on top and gets packed down on top of it, with the sand underneath doing nothing. The sand has to be reapplied on top, hence more often than salt. And applying sand in advance is worthless (well, you can say you did something, but look out in court). Salt or solution can be applied in advance and the snow/ice fall starts melting upon contact. (assuming temperatures are within range for the salt or that solution)
Solutions spread finer, hence have a larger ‘instantly melting’ area and therefore work faster, and can be tailored somewhat to a varied temperature range.
In Minnesota we have had automated bine systems for quite a while and they do work, http://denethor.wlu.ca/pc300/projects/library/anti_icing10.pdf
Have any information on the maintenance costs (its been 17 years now) i would like to know how they stack up against fleet maintenance of sprayer trucks.
No specific dollars on the cost, but my friend’s business was the machines for salting sidewalks and pedestrian walkways. With his equipment, and the city’s larger equipment for the roads, the salt/sand/salt-sand spreading equipment would rust out significantly faster than when used exclusively for various “sand” medias. When they started with spraying the solutions, it was worse. And this is with equipment painted with various ‘chemical resistant’ catalyzed polyurethane paints. With that initial experience, then learnt to be careful when filling the tanks, and changed up where the sprayer nozzles were positioned on the equipment. Can’t tell if salt vs. solution is worse for rusting out the vehicles driving on the roads, as rust protection for vehicles has improved so much over the years.
Minnesota salts their roads to cause vehicles to die of rust.
Then more vehicles need to be built, using iron ore from the Iron Range, which keeps Minnesotans employed!
B^)
I mean…salting roads and walkways is definitely something that is done. It isn’t as good as calcium chloride, but it’s extremely cheap.
How do you think sand is going to melt ice? Let alone snow?
It doesn’t. It just gives you more grip. In the spring it makes the road surfaces thaw quicker because the darker grit catches sunlight.
The problem with salt is manyfold.
1. slowly poisons the environment as it runs off the roads
2. all the cars and trucks rust like heck
3. the salt melts the snow, but unless you’re spraying continuously it soon dilutes and the molten water forms an ice shell
4. salt only really works around 0 C. Below about -5 C it doesn’t work well enough to completely melt the ice/snow and it starts to accumulate
5. people in colder climates have to drive with studded tires anyways, and melting the surface exposes the blacktop to unnecessary wear and tear. That’s why they don’t salt the road, but instead plow/shave it flat, and leave a bit of ice with grit on top for the running surface.
Especially for point 3.
Suppose your road is covered in snow and you spray salt on it. As the snow melts, the salt dilutes and the melting point rises. Unless you spray enough salt to completely melt the snow, it’s going to find an equilibrium where the salt is so dilute that no more snow will melt.
Then you have the roadtop covered in watery slush with deep tire tracks going whichever way. Now the temperature drops by 1 degree, and the whole thing freezes in place. Not good. You have to plow it off before it sets down like hard concrete, which essentially means you salted it for nothing because you still have to clear the road.
@Luke
1) Yup! There are better alternatives for that reason. Those without city/state/national budgets might not be able to afford them for their own personal drive and walkways though.
2) Right again! Coming from an environment with icy winters I am amazed when I travel to warmer climates and see beautiful looking non-rusty cars that are year/models that would be rare rustbuckets if seen at all around my home town. But.. the damage from salt is still far slower and more acceptable than the damage from hitting another car, tree, house, etc…
3) Salt is for ice, really light snows and maybe for slowing accumulation during a snow shower. The correct treatment for accumulated snow is still a plow. Follow this with salt or some other substance to melt the ice and thin snow layer that is left behind. With this small amount of water evaporation will prevent the refreezing problem.
4) The warmer it is the faster the salt works. Hmm.. imagine that. Still, see my reply to #3. Follow that and the snow/ice will not accumulate. Or did you mean the salt accumulates? If the road is already salty you don’t have to salt it again!
5) There are millions of miles of roads driven upon by billions of people that are not icy enough to protect the road from studded tires. And yet without some form of treatment they are still icy enough to cause an accident. Studded tires are illegal here.
Many issues with salt, but the biggest benefit is it saves lives. Until you have a better, or close-enough, alternative, they’ll use salt.
Your point #5 only works in some environments. Each area is different. The temperature ranges will determine what is best on which days.
And no one I know of relies upon ice/solution to remove snow – removing some snow is a limited bonus. Salt/solution is to treat/prevent ice after the bulk of the snow is removed from the surface. The holes salt puts in packed snow also lets vehicles break it up, where it mixes more and melts more until it is either gone or is gathered up with the next plow.
Where I live, it’s illegal to dive with studded tires. Too many bare patches (and often most of the roads) throughout the season (damaging surface, projectile studs)(confess to having a set of Fat Bike tires with studs…). I can encounter snow, packed snow, ice, wet road and dry road, all within one 20 minute drive.
Same for which type of Severe Weather tires work best: snow tire, ice tire or a ice/snow/wet tire. My ice/snow/wet tires on my 4×4 at 40 kph will stop me in: 1.5 car lengths on packed snow, 2.5 on ice, 8ish on black ice. Just 20 kph on cold light fresh scattered snow on black ice and the car is at serious risk of becoming a curling stone, at 10 to 14 car lengths to stop. This is why we use salt.
It all depends on each environment. There is no one best solution.
I’ve lost two cars to salt. Fighting the rust battle with another. I wish we didn’t need to use it. I can empathize (emphasize) with the guy who wigged out and took a shotgun to the salt truck as it went by.
Salt is used all over, ofcourse pedestrians an bicyclist should have it too, it’s one of the most wonderfull things to have happen in my city for bicyclists. I bicycle all year round on mostly perfect condition bicyclepaths at temprature down to -20°C, and moderate snow (5cm to 80cm per day maximum).
Brine solutions works at -15°C to -19°C, at those tempretures it’s more about preventin ice forming rather melting already existing ice. This is from personal experience with 20km of brushed and brined bicycled paths in Sweden. Further I never need to ride with studded tires.
like so:
https://www.youtube.com/watch?v=WMjK4l3LD-Y
@Haista Paska – Melting icy roads and walkways with salt is a well proven method with generations of history behind it. Granted, after a large snow shower it is not the best way to get rid of accumulated snow. Plowing and shoveling do that. Salt treats the ice that is left behind after plowing as well as ice from freezing rain or other damp conditions that are less than a snow shower.
Great way to destroy cars.
Better to do this with a 555.
We were using calcium chloride and sugar beets on our streets. It is less damaging to the environment yet alone our vehicle infrastructure. They went to using salt again after many years without. A friend in the auto maintenance trade said brake line rust-through cases went way up (including mine) after the change-back. Nobody wants to have their brakes break!
You need to wash your car in the autum and then spray transmission oil under your car and then drive dusty sand road. Or just get proper rust protection for your car.
And wash your car on a monthly basis, being sure the underside gets thoroughly washed.
The Raspberry Pi generates enough heat to melt the ice.
An old concept for Germany. Since 1982. ;-) We will keep our Autobahn ice free at hills and bridges.
The nice German word is “Taumittelspühanlage” (melt medium spray facility?) :-)
Some German information (please use google translate):
https://www.strassen.nrw.de/strassenbetrieb/winterdienst/innovationen-im-winterdienst/taumittelspruehanlagen.html
https://de.wikipedia.org/wiki/Taumittelspr%C3%BChanlage
TCO calculations, there is a english summary at the bottom:
https://www.bast.de/BASt_2017/DE/Publikationen/Archiv/Infos/2007-2006/10-2006.html
DHT22?! The humidity reading has an extremely high offset and sometimes completely useless. But I guess it’s fine if you just use them on the street instead of on an automatic weather station.
The problem with this idea is not its methodology but its deployment. You see in order for these automated systems to work there must be stationary nozzels dispersed every x ammount of meters which would raise the price of road laying as well as incur a considerable amount of cost retrofitting it to existing roads. You would have to think about supply (where are the storage tanks for this brine going to be?) as well as delivery (what happens when the brine in the hoses gets below the freezing point in the spray nozel?). That is even before you consider maintenance (how many nozels are there going to be and what is the average maintenance cost per nozel?).
This problem has already been solved by using trucks with plows on the front and brine sprayers or media spreaders (sand, salt or a mix) on the back. The maintenance cost of a fleet of trucks would be much less than stationary nozels while not requiring an increase in future infrastructure costs or retrofitting costs. The trucks would also be usable during the spring,summer and fall months for hauling stuff and other public works uses.
The problem with most universities is that they focus on the science and engineering so much that they rarely teach the students to evaluate if the project is better than the current alternatives.
You forgot to mention that the salt will destroy its delivery mechanism after a few years.
maybe the cheap ones, the good ones are made from stainless. The same as a brine delivery system would have to be or otherwise the delivery system would rust as well.
I could see something coming from this for the auto treatment of bridge surfaces. As I saw in southern Arizona, the roads didn’t need treatment but a number of select bridges’ surfaces did. Instead of sending out a truck based on the forecast, with icing conditions may or may not actually occur, an auto system that treats immediately when required has benefits. No delay; only when needed.
And universities always of tons of projects where the end result is not a useful item/system, nor even expected, but the knowledge gained along the way, and pieces of a project that can be effectively re-purposed or integrated into a viable solution for a similar or entirely different problem. I’m sure you can come up with way ‘worse’ examples of questionable projects. I remember an award given for a university team’s device to be installed in public washrooms that would allow you to open the door with your foot (for no germ contact). It was also a trip hazard; and anyone falling on it was at high risk of a seriously deep brain case puncture… but why let that get in the way of an award.
It actually makes sense on bridges now that i think about it mostly because the support structure is exposed and maintenance would be easier and not require digging into the ground and tearing up the roadway.
So, how does paving the road surfaces with Raspberry Pi’s prevent icing?
I would suspect they would soon be crushed under the tires, sort of like the solar roads…
B^)
Maybe we should be deploying of of those crypto-currency miners along side the roads with underlying heat-pipes to melt the snow & ice away.
Bit of a bust in summer, unless you run the heat-pipes in reverse and use the sun-baked roads’ heat to generate electricity for the mining.
;)
They should just require all the engineering and CS students to put their crypto currency mining rigs in vaults under the sidewalks…