Affordable solid-state batteries large enough for cell phones and drones have been promised for a long time but seem to always be a few years away from production. In this case, Taiwan based Prologium sent [GreatScott] samples of their Lithium Ceramic batteries (LCBs) to test, and even though they’re not yet commercial products, who are we to refuse a peek at what they’ve been up to? They sent him two types, flexible ones (FLCBs) and higher capacity stiff ones (PLCBs).
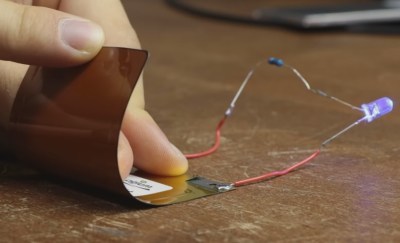 The FLCBs were rated at 100 mAh and just 2 C, both small values but still useful for wearables, especially given their flexibility. Doing some destructive testing, he managed to keep an LED lit while flexing the battery and cutting away at it with tin snips.
The FLCBs were rated at 100 mAh and just 2 C, both small values but still useful for wearables, especially given their flexibility. Doing some destructive testing, he managed to keep an LED lit while flexing the battery and cutting away at it with tin snips.
Switching to the thicker 7.31 Wh PLCB, he measured and weighed it to get an energy density of 258 Wh/L and a specific energy of 118 Wh/kg, only about 2/3rds and 1/2 that of his LiPo and lithium-ion batteries. Repeating the destructive tests with these ones, the LED turned off and smoke appeared while cutting and hammering a nail through, likely due to the shorts caused by the electrically conductive tin snips and nail. But once the snips and nail were moved away, the smoke stopped and the LED lit up again. Overcharging and short-circuiting the batteries both caused the solder connecting the wires to them to melt but nothing else happened. Rapidly discharging through a resistor only resulted in a gradual voltage drop. Clearly, these batteries are much safer than their LiPo and lithium ion counterparts. That safety and their flexibility seem to be their current main selling points should they become available for us hackers. Check out his tests in the video below.
Meanwhile, we’ll have to be content with the occasional tantalizing report from the labs such as this one from MIT of a long battery life and another from one of the co-inventors of the lithium-ion battery which uses a glass electrolyte.
















>”smoke appeared while cutting and hammering a nail through, likely due to the shorts”
That’s always the issue why you can’t make a safe lithium battery. The short circuit itself is dangerous, unless the internal resistance of the battery is too high for the current to rise, which excludes the battery from a majority of applications.
Theres very few applications where you have very small power consumption AND need a rechargeable battery, because primary cells come with much higher energy densities (8-10x). Some IoT sensor might even have a low self-discharge capacitor for the rechargeable part, and simply use the battery cell for a backup.
“because primary cells come with much higher energy densities (8-10x).” I can only relate those numbers by comparing the crappiest NiCad to the type of Lithium Alkaline cells that nobody buys because they cost as much as a decent meal.
Well, this one’s at 118 Wh/kg. Lithium thionyl chloride cells come at 700 Wh/kg and lithium air cells used in hearing aids reach 1600 Wh/kg.
“That’s always the issue why you can’t make a safe lithium battery. The short circuit itself is dangerous”
Nothing is 100% safe but this is many many times safer than other battery lithium based batteries. Ensuring that the battery won’t cause a thermal runaway reaction when damaged is enough to consider it safe for almost every application.
Lithium still burns in air and water, and many of the other materials in the battery such as the carbon in the electrodes are combustible when heated to ignition. In other words, the battery can still go up in flames. It’s just “less volatile/flammable”.
But if you think about where you really need such batteries, like in electric vehicles where you want safety against impacts, you also need very high dis/charge rates, which means very low internal resistance to tolerate very high currents, which have the effect of vaporizing whatever is shorting out the battery and sustaining a plasma arc which turns the materials into a rapidly expanding cloud of hot gas.
Thats pretty negative. Anything will burn, or at least become real nasty to handle, if you get it hot enough.
The reason current technology Lithium batteries burn is because of the electrolyte, not the lithum. The lithium is stored as ions in the electrolyte, not as a raw metal.
Thats the reason that although these cells are still “lithium” cells, they don’t burn when they get cut open.
Some weather stations use just that, supercapacitor that is charged via solar panel and CR2 battery as backup.
The most interesting bit of Info is missing: How do WE/I get one of those cells ?
I checked on prologium’s site and they only talk about the advantages and applications.
Where do I go to buy them (in small quantities) ?
“they’re not yet commercial products”
“Repeating the destructive tests with these ones, the LED turned off and smoke appeared while cutting and hammering a nail through, likely due to the shorts caused by the electrically conductive tin snips and nail. ”
Interesting thing about biological energy sources. They don’t go up in smoke when cut, or hammered.
But they often don’t work at all after being cut or hammered.
Oh, they still work, they just loose their temper.
+1
If they have a comparable energy density and discharge rate to lipo, you better believe there will be the potential for smoke and fire. Energy is energy, it doesn’t matter where you source it.
I don’t think we have any bio-batteries which are up to snuff to output that kind of power and see what happens. Unless you count gasoline, which is organic at least. We know how that kind of energy density works out.
And how well it burns!
This represents a very small subset of the tests per UL1642 and UL2054; which is more than can be said for most Li battery packs out of China, so is interesting. The safety of this Li-ceramic stuff would seem to be promising.
But would have been useful to have done charge/discharge curves over the range of rated operating temperatures prior to the mechanical/enclosure tests.
May I be the first to say: Shut up and take my money!
They would definitely be far safer for use in vehicles. No fires or explosions if damaged in a crash.
Size, capacity and weight are still very high on the “concerns” list.
These batteries have a solid electrolyte. They are not solid state. There is still a significant volume change of the electrodes from charge to discharge. Solid state is electrons and holes, and no volume change.
/soapbox
Geez he tested it by destroying it that’s original….
The major feature of this chemistry is safety. So it makes sense to do this type of test.
Sounds almost exactly like the claims that were made for lithium polymer batteries about 20 years ago.
The flexibility and wearability. So, I imagine an entire duck bill hat made from the stuff with a heat sinked cree xpl (200 lpw) run at 2 watts. That could go a week between charges.
Then you toss it in the dryer by accident and it burns down your house.
Not entirely on topic, but doing those kind of tests in rubber gloves where heat is produced is not the best idea.
Where can i get a Lithium Ceramic battery?
What about low temperature? How will this affect the performance?
I like how thin they are, would be very useful for portable devices like a portable Wii in a thin case, since the problem has always been big batteries equals big case. Looks like you can stack like 20 of those FLCBs in the same thickness, that’s awesome!