Ensuring that water is safe to use and consume can be a real chore, especially for those who live in impoverished areas without access to safe drinking water. Here is where researchers at Stanford University hope that their recently developed low-cost catalyst can make a difference. This catalyst comes in the form of nano-sized particles (nanoflakes) consisting out aluminium oxide, molybdenum sulfide, copper and iron oxide. When exposed to sunlight, the catalyst performs like a photon-sensitive semiconductor/metal junction (Cu-MoS2), with the dislodged electrons going on to react with the surrounding water, resulting in the formation of hydrogen peroxide (H2O2) and hydroxy radicals.
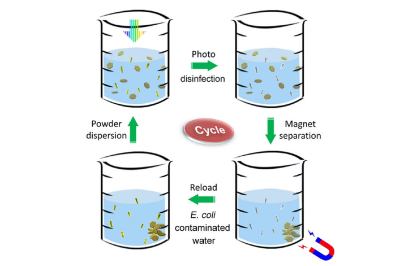
Waterborne diseases are very common, with even the US reporting 7,000 deaths and 120,000 hospitalizations in 2021, according to the US CDC, and many more affected worldwide. Much of the harm is done by microbes, in particular bacteria such as E. coli, which are prolific in aquatic environments. By using this catalyst powder in contaminated water, the researchers reported that the Escherichia coli colonies in the tested samples were fully eradicated after a 60 second exposure to sunlight.
The reason for this is that hydrogen peroxide and similar reactive oxygen species are highly destructive to living cells, yet they are simultaneously very safe. Because of their high reactivity they are very unstable and thus short-lived. This is useful when the water with the now very dead microbes is consumed afterwards, with the catalyst itself being ferromagnetic and thus easily separated using a magnet.
With this proof of concept in hand, it’d be interesting to see what the product will look like, especially when it comes to the final separation step and making this as easy as possible. Since the catalyst is not consumed or presumably contaminated, it can last pretty much forever, making it an attractive alternative to water purification tablets and expensive filtration systems.
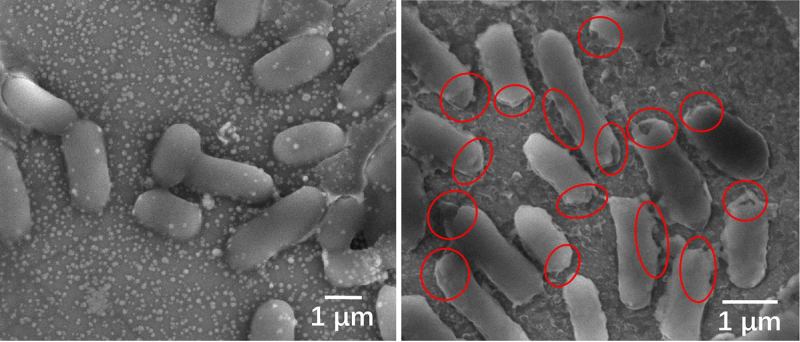
(Heading image: Microscopic images of E. coli before (left) and after disinfection. The bacteria died quickly after sunlight produced chemicals that caused serious damage to the bacterial cell membranes, as shown in the red circles. (Image credit: Tong Wu/Stanford University) )












About hydrogen peroxyde, beside microbes/bacteria, can it also kill fungus, especially spores? How long would it take, and what concentration is recommended?
If not, one could use 222nm far-UVC instead
Distant memory of silver or silver nitrate killing bacteria, is this so or is my memory failing.
No you’re quite right – and you’ll see it incorporated in athletes’ apparel for that reason.
I see, I was once involved in a hush hush project to coat filament with a mysterious coating, (silver based) my job was to build an on demand heating process to cure the coating just prior to entering the extruder, my experiments proved that a uv laser worked far better and more controllable than cartridge heaters, but this was rejected as funding specified cartridge heaters. The concept being complex 3d structures that sterilize and possibly antenna.
100ppm H202 or 10ppm silver stabilized H202 when watering can help get it under control. Silver helps the stability of the H202 molecule and has its own beneficial anti-x properties.
Usually mold is from high moisture & low sunlight. Time to kill the spores will be forever without correcting these. If you have lots of moss you probably have these issues.
There are other natural options like using Bacillus (bacteria) but the same moisture and sunlight issues still apply.
Mold spores can survive pretty extreme temperatures for years.
100ppm H202 or 10ppm silver stabilized H202 when watering will help keep the mold away. Bacillus (bacteria) can also work well (but not with H202 and isn’t compatible with some plants.) Silver helps stabilize the H202 molecule to stick around and has its own anti_ properties.
If you have a moss issue chances are also higher for having mold issues. Sunlight and fixing moisture / drainage issues are both very important in combating mold. Without fixing those the peroxide won’t help long term. Definitely worth focusing on those of you have a mold issue.
Mold spores can survive extreme temps and can stick around for years.
What happens to the Hydrogen Peroxide and hydroxy radicals after the bacteria are killed? They just become water again?
Yup
Thanks! So a reversible reaction (albeit not for the bacteria 😉 )
I saw a thing once where they built a frame to hold PET (I think?) bottles of water so that you could let the UV kill the microbes directly. It took a lot longer than 60 seconds though, more like hours.
Yes, it’s called Solar Water Disinfection (SODIS).
https://upload.wikimedia.org/wikipedia/commons/6/67/Indonesia-sodis-gross.jpg
Didn’t the former president suggest you could kill the Covid virus with sunlight, too?
Yes, UV exposure kills/inactivates most pathogens. Far-UVC safely (for humans) kills airborne pathogens and copper alloys used, for instance, for door handles also kill pathogens: “The process involves the release of copper ions (electrically charged particles) when microbes, transferred by touching, sneezing or vomiting, land on the copper surface. The ions prevent cell respiration, punch holes in the bacterial cell membrane or disrupt the viral coat, and destroy the DNA and RNA inside.”
Yes, but you need large quantities of orange KoolAid for it to work.
As opposed to the blue state Kool-aid you consume regularly?
It’s all the same kool-aid, they just put different food coloring in every four years.
It beats drinking bleach magat
Both of you should stop caring about politicians. The ruling class certainly doesn’t care about you.
but we certainly care about them…
You can, to an extent. The 24 to 48 hour surface suruvival times initially suggested were for idealised indoor conditions, outdoor sunlight limits the surface survival types of common respiratory type viruses to minutes. Fresh air and strong sunlight is certainly far healthier than hiding at home getting fat in stuffy air.
The GBD is just yet more trickle down economics masquerading as health advice.
The GBD is simply a statement of what all scientists in public health agreed in 2019 (and beforehand) until suddenly panic (a “the day we thought the dam broke in Columbus Ohio” type event when scary news appeared on the horizon) over-took too many of them. GBD’s authors have no sympathy for nonsense like trickle-down economics (wealth doesn’t trickle down, it trickles out when the super-rich evade taxes and send the wealth abroad), Sunetra Gupta is even a bit of an anti-capitalist. The GBD is sensible common-sense advice for how to improve health overall rather than fixate on one single disease which posed a hazard almost solely to the very elderly. Totalitarian restrictions have killed people through making the poor poorer while the super rich got richer, poverty massively shortens life expectancy, and all that restrictive NPIs did was drive workers and small business owners in to poverty whilst mega-corporations kept running and made several new billionaires. President T was an idiot indeed, but Scott Atlas (a GBD signer, though not one of the original authors) was very wise despite having the misfortune of having worked or the-orange-one for a short while.
Somehow. treating the reasons water is polluted in those places could have more permanent benefits ….
We can do both.
How is aluminum oxide and copper removed with a magnet?
Found the answer…. These are not just individual ingredients in a mixture. The ingredients form a nano-structure with the given properties. This information is in the abstract (linked in the nature article). Unfortunately, the rest of the paper is behind a paywall.
Kinda have to wonder whether by now things as important as sustainable water purification research really ought not be behind a paywall…
Paywalls aren’t real, use Sci-Hub.
“Much of the harm is done by microbes, in particular bacteria such as E. coli, which are prolific in aquatic environments.”
Interesting there isn’t more dead animals then. Those that live in it, and those that drink it.
Yeah, one of my pet peeves is saying “E. coli is bad”. It’s like saying “gasses are bad” because breathing chlorine might kill you or “animals are bad” because someone got bit by a shark. There are so many strains of E. coli that are beneficial, the author may as well say “E. coli is good”, too.
Next to add a molecular sieve and some souped up hemoglobin, to grab the hydrogen and oxygen before they recombine.
Can it generate peroxide at a high enough concentration to be useful as an antiseptic? If not directly, perhaps the peroxide can be concentrated or separated somehow to do so.
Wasn’t that an argument for making all hospital door handles, drawer handles, etc, out of brass or another copper alloy? They self-sanitise.
I wonder if you could make a specialised bottle that contains the catalyst, as well as an electromagnet around the sides. Pour your water into the bottle, shake it up to distribute the catalyst, then after a minute, wind a handle to generate some power into the electromagnet to draw the catalyst to the sides of the bottle. (Or even just around the neck of the bottle, since the electromagnetic field would be strongest there?). Pour your water out, and repeat.