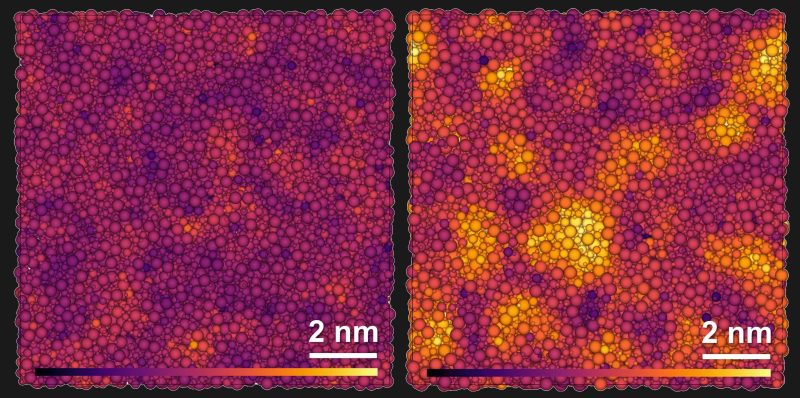
There’s some nifty research from the University of Bayreuth, together with partners in China and the U.S., on creating supremely tough aluminosilicate glass that boasts an unusual structure. The image above represents regular glass structure on the left, and the paracrystalline structure on the right.
Aluminosilicate, which contains silicon, aluminum, boron and oxygen, is a type of oxide glass. Oxide glasses are a group to which borosilicate and other common glasses belong. Structurally speaking, these glasses all have a relatively disordered internal structure. They’re known for their clarity, but not especially their durability.
One thing scientists have learned over time is that unusual things tend to happen under high pressures. In this case, researchers discovered temperatures around 1000°C and pressures between 10 and 15 gigapascals (a tremendous but not absurd amount of pressure) caused silicon, aluminum, boron and oxygen atoms to group together in a way that is clearly not fully crystallized, but is still different from the usual irregular structure of glass. This paracrystallization remains even after pressure and temperatures drop.
What does this mean? It means a toughness never before recorded in oxide glasses, and it doesn’t even really affect how clear the glass is. When subjected to forces that would normally cause cracks or fractures, these stresses are instead absorbed by the paracrystalline structures, which themselves transform back into the amorphous, random structure more usually seen in glass. Check out the research paper Toughening oxide glasses through paracrystallization to learn more.
Despite the name, aluminosilicate glass isn’t transparent aluminum. This may not be something one will do in a home workshop anytime soon, but it’s still pretty nifty.














“They’re known for their clarity, but not especially their durability.”
I thought Pyrex[tm] was borosilicate.
Yes! Old school Pyrex is a Corning brand name for borosilicate glass. Modern pyrex (lower case) is an Intstant Brands brand name for tempered soda-lime glass cookware.
The most familiar brand name aluminosilicate glass is probably Corning Gorilla Glass.
Sapphires and rubies are an aluminium oxide Al2O3 and both transparent and very tough, if crystalline and not a glass.
So THOSE are transparent aluminum, then. Right?
Yes and no. Glass means multiple things in multiple areas, for example “glass” on your watches is not glass as chemists / physicist categorize glass. But we call it glass anyway….
I never mentioned glass. Talking about sapphire and ruby.
Scotty is real! haha ;-)
It depends where you are too. European Pyrex is currently borosilicate glass, I expect that can change as the market and supply changes.
Borosilicate glass, including borosilicate Pyrex, is extremely resilient to thermal shock, but less resilient to mechanical shock (for instance dropping it).
“these stresses are instead absorbed by the paracrystalline structures, which themselves transform back into the amorphous, random structure more usually seen in glass.”
So the second time the stress happens it’ll just break in those places?
Same question.
The other end of the link says “… forces acting on the glass from outside, which would normally lead to breakage or internal cracks, are now primarily directed against the paracrystalline structures. They dissolve areas of these structures and transform them back into an amorphous, random state. In this way, the glass as a whole acquires greater internal plasticity, so that it does not break or crack when it is exposed to these or even to stronger forces.”
…which makes it sound like turning back into mundane glass makes it even stronger. ?.
Sounds like something is getting lost in summarization.
The journal article is paywalled except for the abstract which isn’t much more helpful: “We attribute this exceptional toughening to the excitation of multiple shear bands caused by a stress-induced inverse transformation from the paracrystalline to amorphous states, revealing plastic deformation characteristics.”
Are they saying the de-crystallized form gets plasticky?
Plastic deformation means it does not come back to its previous state when the stress is released.
The abstract clearly says it gives you one more chance and that’s it; )
So, good for stopping a bullet, not so good for stopping a full metal jacket.
understood re plastic deformation.
I’m not seeing the “clearly says” part.
It doesn’t sit right that the authors called a one-and-done material “tough”.
It is just standard old school forging, you do it at higher temperatuire in this case because of material choice. Same principles apply here.
Sounds like transparent aluminum to me!
That’s exactly what it is!
it’s “transparent aluminium” for people that mix up silicon and silicone :)
Compounds of aluminum are not aluminum any more than water is hydrogen or people are carbon. Please stop pretending that space opera is reality.
I reject your reality and substitute it for my own!
Wow—- Bad day there???
Maybe burn one and chill??
A certain sub could have used this for their windows.
Sounds like it won’t break on the first bang, but the first bang causes it to lose its strength. So it will break on the second bang.
Did I understand that right?
yes. It will be ok against an errant baseball, or a rock thrown from a tire, but not Maxwells silver hammer. At least not locally.
So if it’s hit hard enough to crack will it explode like a Prince Rupert drop?