All of us have to deal with the looming threat of developing cancer during our lifetime, no matter how good our genetics are, or how healthy our lifestyle is. Despite major improvements to the way that we treat and even cure cases of cancer, the reality today is that not all types of cancer are treatable, in many cases there’s the likelihood that one day it will return even after full remission, and chemotherapy in particular comes with potential life-long health issues. Of the most promising new and upcoming treatments, immunotherapy, is decidedly among the most interesting.
With this approach, it is the body’s own immune system that is taught to attack those cancer cells, requiring little more than a few tweaks to T-cells harvested from the patient’s body, after which they’re sent on their merry cancer-killing way. Yet as simple as this sounds, finding the right characteristics which identify the cancerous cells, and getting a solid and long-lasting immune response is a tough challenge. Despite highly promising results with immunotherapy treatment for non-solid cancers like leukemia – that have resulted in almost miraculous cures – translating this success to other cancer types has so far remained elusive.
New research now shows that changing some characteristics of these modified (chimeric antigen receptors, or CAR) T-cells may be key to making them significantly more long-lived and effective within a patient’s body. Is this the key to making immunotherapy possible for many more cancers?
No Two Alike
Important to note about cancer is that it is not the name for a singular disease, but rather the collective name for a wide range of diseases, all of which involve abnormal growth with the potential to invade or spread (metastasize) to other parts of the body. This contrasts such growths with benign tumors, which are still potentially problematic if they end up exerting pressure on blood vessels, nerves or organs, but which are sufficiently differentiated from the surrounding tissue that they can be surgically removed if necessary. Some of these benign tumors can later become malignant, which is why even very common tumors like a melanocytic nevus (‘mole’) ought to be paid attention to in case of any changes.
In the case of a malignant tumor we thus call it collectively ‘cancer’, which can affect one or more organs and/or tissue types within the body. There are many reasons for why a cell can become cancerous, ranging from exposure to carcinogens, as found in cigarette smoke and other forms of pollution or in certain diets, all the way to genetics (e.g. BRCA1 and BRCA2 mutations for breast cancer) and a viral infection as well as hormones and ionizing radiation exposure. Some types of cancers are caused by exposure to minuscule inert physical elements, like asbestos.
With so many ways that a cancerous growth can start, it is perhaps more interesting why we aren’t developing fresh malignant tumors every single month. There are a number of answers to this, involving both built-in mechanisms in the body’s cells that detect when something isn’t right, at which point apoptosis (programmed cell death) will be triggered. Apoptosis can also be triggered externally using receptors on the cell membrane to which a cytotoxic T cell connects. Using these intrinsic and extrinsic apoptosis pathways cells generally either terminate themselves, or are identified as malignant by the body’s immune system and induced to terminate.
So why is it that some malignant tumors manage to develop regardless of these mechanisms?
T Cell Exhaustion
As effective as the body’s adaptive immune system is, it has a few weaknesses. One of these is a phenomenon called ‘exhaustion’, whereby chronic antigen exposure causes T cells to lose their effector function and transition first into a ‘precursors of exhausted’ (TPEX) form before the terminal differentiation into ‘exhausted’ (TEX) form. These TPEX cells can then neither fight against the infection or tumor (as T effector, or TEFF), nor become a T effector memory cell (TEM) later on, nor produce more T cells. As rapidly growing malignant tumors are likely to flood the body with antigens, such exhaustion is quite likely to occur.
Although TEFF cells are replenished by core memory cells (TCM) and even less differentiated stem memory cells (TSCM), this is not sufficient to counteract the cancerous cells. This explains both why sometimes a malignant tumor can progress essentially unimpeded and why T cell-based immunotherapies have had little luck treating solid cancers as there simply aren’t enough active TEFF cells present.
This raises the questions of why T cell exhaustion exists, and whether disabling this mechanism in select T cells might offer a solution.
Epigenetic Expressions
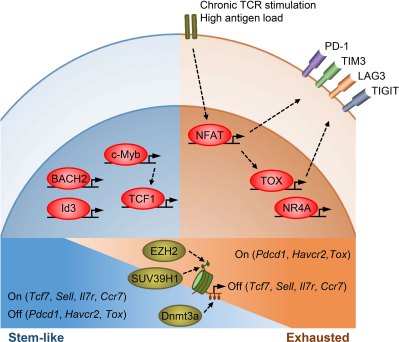
The mechanism that changes TEFF cells is that of epigenetics, specifically through the methylation of the cell’s DNA, not unlike the methylation-induced aging of the body’s cells as a whole, a factor which has been shown to also play an important role in tumorigenesis. As suggested by Enyong Dai and colleagues in Molecular Cancer, preventative therapies against cancer could involve epigenetic drugs which would address such underlying epigenetic changes in addition to directly treating existing malignant tumors.
Epigenetics is an essential element of cellular functioning, which includes DNA methylation and structural elements like chromatin and its histones, by regulating genetic expression. It also enables generation-level evolutionary changes since aspects like methylation are hereditary. Here DNA methylation is also the most relevant in how it directly affects the individual, especially as it pertains to TEFF cells.
As detailed by Xuechen Yin and colleagues in a 2023 review article in Immunology, TEX cells are characterized by overexpression of inhibitory receptors (such as programmed death-1, or PD-1), with TPEX cells able to be revived through blocking these receptors. In addition, CRISPR-Cas9 was used to create PD-1 knockout CAR T cells, which demonstrated significantly better performance against solid tumors. Here a potential target has been longer known, in the form of methylation of histone 3 lysine 9 (H3K9) and its effect on modulating immune cell differentiation and immune response. This epigenetic mark is thus highly influential in the outcome of events where the immune system is involved.
The role of histone methylation in immunotherapy targeting tumors was discussed in detail by Yuanling Zhang and colleagues in a January 2023 review article in Frontiers in Immunology, providing an overview of the different pathways within both T cells and malignant tumor cells that affect immunotherapy. One of these mentioned is the gene SUV39H1, which affects the methylation of H3K9me3 and suppresses the killing and memory functions of TEFF cells.
Perhaps unsurprisingly, it is this exact SUV39H1 gene that Nayan Jain and colleagues in this newest research as mentioned earlier have targeted when they used CRISPR-Cas9 to create SUV39H1 knockout CAR T cells. In trials these knockout CAR T cells were repeatedly challenged with antigens, but did not show the usual signs of turning from TEFF cells into TPEX or TEX cells. Effectively this would appear to have removed at least the most effective pathways by which T cells can become exhausted, and trials on mice have shown encouraging results.
A Long But Promising Road
Although immunotherapy is an approved therapy for a number of forms of cancer like leukemia, the T cell exhaustion issue has so far been one of the main obstacles in its wider use. With clinical trials of SUV39H1 knockout CAR T cells being a distinct possibility at this point, it could be only a matter of years until we begin to see the effects of this particular approach with CAR T immunotherapy.
Perhaps it’s not so surprising that with sci-fi literature and movies often referencing ‘medical nanobots’ that we would end up at this point where we would do our best to reverse engineer the nano machines that already exist within our own bodies. Courtesy of a rapidly growing body of medical literature and advanced gene-editing tools at our behest we can today dream of cures and therapies that would have seemed like the purest sci-fi only a few decades ago.
With how terrible cancer is, and how many lives it claims every single day, it almost seems wrong to imagine a world in which every type of cancer is not only treatable, but also curable through routine immunotherapy. Yet this might be the future on which cusp we now find ourselves.

















Absolutely fascinating info. I’d read about this treatment a few years ago but didn’t grasp some of the finer points like the different T-cell states. This seems like such a promising line of inquiry that I wish I could help. Thanks for the great article!