How good are ion membrane dehumidifiers for keeping FDM filament dry and ready for printing? This is the question which [Stefan] at CNC Kitchen sought to answer in a recent video. Like many of us, he was inspired by a video which [Big Clive] made a while ago in which said dehumidifiers were demonstrated for keeping an enclosure free from moisture. Yet would they be able to tackle the much bigger drying job of one or more spools of filament? Thanks to some free samples sent by Rosahl, [Stefan] was able to start answering this question.
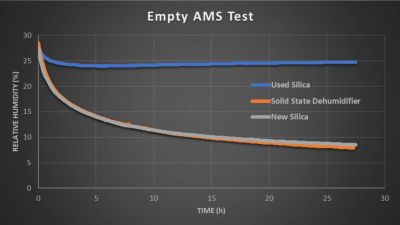
In the experiments, he used the smaller RS1 (€36.25 a piece) for a single spool container, and the larger MDL-3 (€169) with a Bambu Lab AMS multi-spool unit. Normally such an AMS has three big containers with silica desiccant in it that have to be regularly swapped out, but he modified one AMS to only have the big MDL-3 membrane to dehumidify. A second AMS was left with older silica in its containers, and a third got fresh silica, allowing for some benchmarking between the three units.
The results say a lot, with the initial empty AMS test showing the older silica desiccants topping out quickly and leaving the fresh silica and the membrane dehumidifier to go neck to neck. This is not the usual scenario in which you’d use these dehumidification methods of course, and the small-scale test with the RS1 showed that with a full filament spool in the box, humidity inside the container would only drop very gradually as more and more moisture replaced what was removed from the air. In particular the cardboard element of the spool being used was suspected of being one of the biggest sources of moisture.
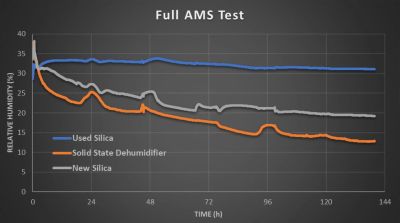
With multiple spools inside the three AMS units to simulate a more typical scenario, the performance was rather similar, except with the used silica’s performance absolute cratering and the impact of moisture replacement in the air being very noticeable for the other two AMS units as humidity kept moving around even as it went gradually down, with the ion membrane dehumidifier taking it down far further than even the fresh silica.
Effectively, this means that such an ion membrane dehumidifier is a good choice, as it does exactly what it says on the tin, with a rather humble power usage of around 4 watt (at 3 V).
This doesn’t mean that heated drying boxes can be thrown out, of course, as they are much faster and it makes sense to first dry spools out before putting them in a storage box with one of these solid state units. The main negatives to such a solid state solution are probably the price and the oxygen accumulation inside the container, as the ion membrane works as hinted at through electrolysis with the hydrogen ions moving through the membrane, which leaves the oxygen atoms behind.

















There are some youtube videos about making ion exchange membranes using napkins, pva glue, cement and some unfamiliar chemicals. Maybe someone can figure out opensource version of this as well…
Has anyone found a good source for these units in the States?
Desiccant can be reused through heating, so I wasn’t clear on his distinction between used vs. fresh. I can see that maybe it might degrade over time, but was his used desiccant heated to release its moisture first in these tests?
Sorry, posted in the wrong spot.
I’d be very interested to know just how oxygen rich the air inside a storage box would get after say 6 months or a year, and if that had any negative impacts on different filaments being stored. If the answer is it’s negligable then this could be a fantastic solution for anyone like myself living somewhere prone to humidity.
If the moisture can get in, the oxygen can get out. In equilibrium the concentration of additional oxygen should match the difference in concentration of water inside vs outside. We are talking an additional 0.1-0.5% of oxygen, compared to ambient which is around 21%
Wonder how close that amount is for spontaneous combustion of non-flammable materials. Around 25% oxygen and fire-proof work clothes will easily burn like a torch.
So an extra 19% oxygen will cause things to easily and efficiently burn (like a torch) that were designed not to burn at all? Not only burn when they shouldn’t, but do so spontaneously which implies the lack of a heat source as well as a fuel (being fire proof)???
Not sure what you’re saying or asking though I’m curious?
Did you mean 25% of the total was Oxygen, or when there was 25% more Oxygen? Actually that’s only a ~1% (total) difference…
Are you sure? Who makes these clothes and where can I read about the protection they claim to provide?
Are you really not thinking the AMS will be opened for 6-12 months??
They seem to claim they will sell these only to “equipment manufacturers with suitably trained staff”.
Kind of cuts into its usefulness if you can’t GET ONE.
The price is probably a dealbreaker anyway. The membrane he tested costed more than the AMS.
I just recived mine, no problem. You just pay through PayPal. From ordering it took 1 week to arrive, BUT remember the taxes from your cuntry will be added.
Right now they sell the for 90£
Why not just use a hydrogen fuel cell membrane? There plenty of cheap educational kits on Amazon and Ebay.
They generally need to be wet to work.
I see they make air dryers out nafion which is typically what’s used in fuel cells. Also https://www.amazon.com/Davis-Instruments-1459-Air-Dryr-Dryer/dp/B000B7MU9Q might be a cheaper and more availble solution.
Unless I’m very confused that’s just a lightweight heater. If you seal the volume there won’t be any reduction in moisture, it’s just that more of it will be in the air rather than on/in the things in the volume.
That’s not exactly bad, but you could do the same thing by turning your heated bed on. Or heck, your computer for that matter. All electric devices are 100% efficient at turning any consumed power into heat, but at least the computer is doing something with it first.
“It works by drawing moist air in, heating it, and then expelling dry air out the top.” Taken from manufacturers website https://www.davisinstruments.com/products/air-dryr-500
Yeah that Davis Instruments thing is literally just a resistive heater. No moisture is removed from the air at all – it just heats the air and thus lowers the relative humidity.
An RO membrane is made of materials that let much more water vapor than air through. The usual housing unfortunately has only one connection on the permeate side, which limits our options for making the water go opposite the usual way. In other words, a permeate sweep gas (room air) is hard.
A vacuum pump might be the best option for making the membrane run. The permeate pressure has to be less than your desired partial pressure of water (say 0.1 – 1.5 kPa). A commercial pump is probably expensive so maybe that part is worth DIYing.
I would add maybe some TEC’s in Peltier configuration to remove heat from the vessel – cooler air does not hold as much moisture as warmer air.
It’s not about removing moisture from the air (in this application).
You want to remove it from the filament itself. So you lower the air moisture content, this allows moisture to leave the filament to the air, which is then removed.
Lowering the temperature means you can’t get the moisture to come out of the filament.
Heating the filament and providing a cold surface for moisture to condense onto could let you do this in a sealed container, so long as you could keep the moisture from evaporating again once it was on the TEC. Capillary action siphon?
I wonder if it could be made like a defrost cycle for a conventional refrigerator? Periodically turned off and the water drip out through a small hole. Or if it doesn’t freeze then it could just drip out on its own without being shut off. A fridge typically has its evaporator obscured to reduce warming of the food, but in this use it might not be needed.
https://www.bhphotovideo.com/c/product/1760420-REG/ruggard_edc_80lc_electronic_dry_cabinet.html I always thought those worked like that. Mine stays at about 10% humidity at present.
Vacuum could “boil” the moisture out of the filament.
Wouldn’t that necessitate a way to remove the moisture once released, to avoid reabsorbstion when vacuum is pulled? Sounds like square uno
More vacuum
I tried this to recharge silica beads, since I have an old Fisher Scientific vacuum oven. It works, but I would *not* recommend it unless your vacuum pump is set up to handle moving moisture (unlike mine). For the basic / cheap rotary vane wet pumps (wet in that they use oil) the moisture is emulsified into the oil and gets into the insides of the pump. Now I have to change and flush the oil lest it rust.
If yours hasn’t got a gas ballast, you could consider getting a needle valve and making your own to help with the water, couldn’t you? You’d want to run it awhile after seeing the pressure drop off, but still. Some of those cheap pumps have one, some don’t.
An alternative is a two-state air venturi or a water aspirator with something other than plain water as the working fluid. The first one would take an incredibly annoying amount of air to actually work, and I managed roughly low enough pressure to chill water, but not low enough to freeze. The second one would only work if you used something with a lower vapor pressure than regular water, like cold water, a concentrated brine, or a different liquid, and I didn’t try. For the silica beads, though, might still be best to just use more heat as long as it’s not so much as to crack them. *shrug*
Wouldn’t that also remove the plasticizer?
Desiccant can be reused through heating, so I wasn’t clear on his distinction between used vs. fresh. I can see that maybe it might degrade over time, but was his used desiccant heated to release its moisture first in these tests?
Used – already had some moisture inside from previous drying runs. Fresh – regenerated just like you said, by heating.
What happens with the oxygen that is left in the box? Water (H2O) is split, the H ions diffuse out and recombines with oxygen on the outside to form water but the singlet oxygen left on the inside is higly reactive, what happens? See for instance https://water.lsbu.ac.uk/water/o2water.html
I was thinking about that – do we now leave a jar of iron shavings in there to soak up oxygen (making rust) instead of the silica beads?
Maybe that’s far less of an issue, but it would be good to test if these ever got cheap and available.
The water in the air may be needed as a catalyst in the slow oxidization of the iron. As for an Iron source, use the finest of steel wool, might even cause a spontaneous metal fire.
My dry box lives in my shop, which is not normally climate controlled. Houston is a hot swamp, and silica beads release moisture on hot days… I put in a thermoelectric dehumidifier, which did a solid job. A light bulb keeps the inside somewhat above ambient. I later upgraded to a 2 stage TEC. It gives me 23-29% humidity despite ambient to 81F!
I’ll stick to my food dehydrator and dessicant.