Fiber lasers aren’t nearly as common as their diode and CO2 cousins, but if you’re lucky enough to have one in your garage or local makerspace, this technique for depositing thin films of metals in [Breaking Taps] video, embedded below, might be worth checking out.
It’s a very simple hack: a metal shim or foil is sandwiched between two pieces of glass, and the laser is focused on the metal. Etching the foil blasts off enough metal to deposit a thin film of it onto the glass. From electron microscopy, [Breaking Taps] reveals that what’s happening is that microscopic molten metal droplets are splashing up to the ̶m̶e̶t̶a̶l̶ glass, rather than this being any kind of plasma process like sputtering. He found this technique worked best with silver of all the materials tested, and there were a few. While copper worked, it was not terribly conductive — he suggests electroplating a thicker layer onto the (probably rather oxidized) copper before trying to solder, but demonstrates soldering to it regardless, which seems to work.
This might be a neat way to make artistic glass-substrate PCBs. More testing will be needed to see if this would be worth the effort over just gluing copper foil to glass, as has been done before. [Breaking Taps] suspects, and we agree, that his process would work better under an inert atmosphere, and we’d like to see it tried.
One thing to note is that, regardless of atmosphere, alloys are a bit iffy with this technique, as the ‘blast little drops off’ process can cause them to demix on the glass surface. He also reasons that ‘printing’ a large area of metal onto the glass, and then etching it off would be a more reliable technique than trying to deposit complex patterns directly to the glass in one go. Either way, though, it’s worth a try if you have a fiber laser.
Don’t have a fiber laser? Maybe you could build one.

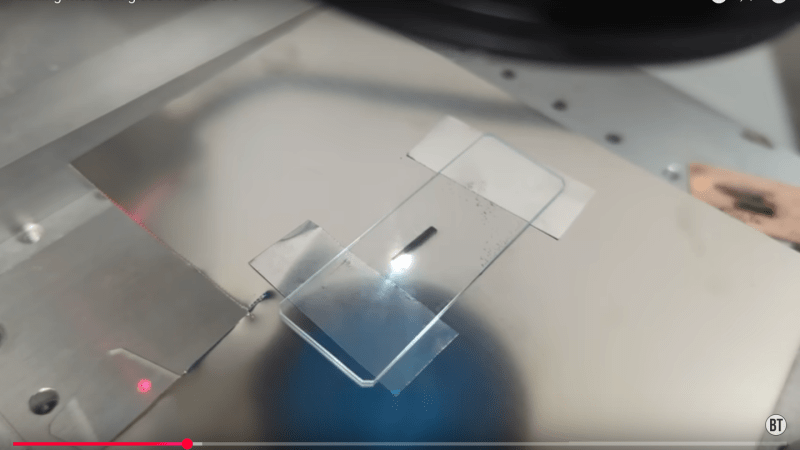














Me want at home
I think that the following sentence might have a typo…
“what’s happening is that microscopic molten metal droplets are splashing up to the metal”
Is that supposed to be droplets splashing up to the glass?
I look forward to digging into this more later, it looks quite interesting.
I think that the following sentence might have a typo…
“what’s happening is that microscopic molten metal droplets are splashing up to the metal”
Is that supposed to be droplets splashing up to the glass?
I look forward to digging into this more later, it looks quite interesting.
Tried this at home as soon as the laser settings were shown in the video. Aluminum really wants to weld to the glass, but brass sheets from the craft store gave good consistent results easily.
You can mark metal through clear acrylic as well, but I couldn’t get the metal to stick to it. I tried because it would’ve been a neat way to make a business card.
That’s interesting, since he specifically had bad results with alloys.
I was thinking of trying this with carbon dioxide as a (hopefully) inert gas, mostly because it’s easy to make, and don’t requires a bottle and regulators.
Pretty surprised how well this works. Usually metals don’t wet or adhere to glass.
Mostly this sort of thing is done with mangetron sputtering or vapor deposition under a vacuum. Thin layers of chromium like 5-10nm help metals adhere.
Neato
It’s probably like spot welding… There is not much oxygen in the thing gap so after brief initial oxidation, it gets consumed rather quickly… Therefore no vacuum or shielding is neccessary…
We did some vacuum deposition of aluminum and silicon with an appropriate stencil to make schottky diodes (and some sort of FET, I believe) in some Physics lab back in my college days. That might also be a fun way to make glass PCB traces. It shouldn’t be that hard in the home lab if you already have a vacuum chamber with suitable electrical pass throughs.
For extra awesome points (a furnace, some sort of nasty HF paste) given a few steps you could literally plate and dope your own semiconductors.
Glass is Al2O3 which is pretty much oxidized aluminium so when it’s heated by laser it gets decomposed back to oxygen and aluminium and it forms a good surface layer for metal to weld to. If it becomes cheaper (like 3D printers) then I can easily see jlcpcb going out of business.
Utter BS all along.
…isn’t most glass silicon oxide based? I’m not aware of any glasses that are alumina based.
I guess as an adative there might be some in some types of glass?
Gorilla glass = pure Al203
Ruby, sapphire = also Al2O3 but with other metal impurities that give the color.
Normal glass (soda-lime) is mostly silica
Some optical glass or lab glass are borosilica
Glass is just a generic term for transparent (in visible spectrum), amorphous material.
Yes glass is absolutely silicon based and not aluminum based.
There are some aluminum oxide based ‘glasses’. You have have amorphous metal glasses, the first discovered ones containing some aluminum.
Great experiment! Having worked on film deposition, I found surface preparation essential. Could you please mention how the slide and foil were cleaned?
Also, have you tried preheating the glass? An increase in temp might reduce surface contamination, improve droplet spread and reduce micro-cracking.
Finally, what about doing this in partial vacuo? Yes, it would increase complexity of that beautifully simple process, but that technique would require pumping out only a tiny volume, and the very short path between glass and metal would reduce chances of hitting gas molecules without requiring extreme vacuum.
My very first thought was could this be used in any way shape or form to mirror home ground glass telescope mirrors. And the more I think about it, the more potential problems I forsee. Getting a consistant nearly constant coating thickness, in the order of nanometers, would be the primary problem.
Can this be used to create silver conductive traces that may be able to transfer from the glass onto another substrate?