For organic semiconductors like the very common organic light-emitting diode (OLED), the issue of degradation due to contaminants that act as charge traps is a major problem. During the development of OLEDs, this was very pronounced in the difference between the different colors and the bandgap which they operated in. Due to blue OLEDs especially being sensitive to these charge traps, it still is the OLED type that degrades the quickest as contaminants like oxygen affect it the strongest. Recent research published in Nature Materials from researchers at the Max Planck Institute for Polymer Research by Oskar Sachnik and colleagues (press release) may however have found a way to shield the electron-carrying parts of organic semiconductors from such contaminants.
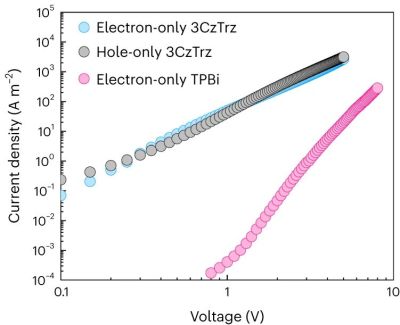
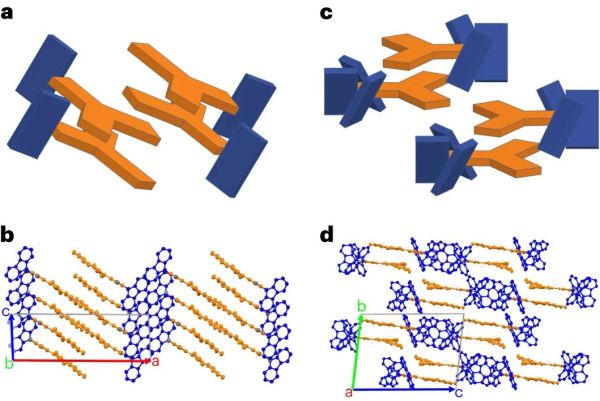
In current organic semiconductors TPBi is used for electron transport, whereas for this research triazine (Trz, as electron acceptor) and carbozole (Cz, as donor) were used and compared with the properties of leading-edge TPBi. While a few other formulations in the study did not show remarkable results, one compound (3CzTrz) was found using X-ray diffraction (XRD) to have a structure as shown on the right in the heading image, with the carbozole (in blue) forming essentially channels along which electrons can move, while shielded from contaminants by the triazine.
Using this research it might be possible to create organic semiconductors in the future which are free of charge-traps, and both efficiency and longevity of this type of semiconductor (including OLEDs and perovskites) can be improved immensely.