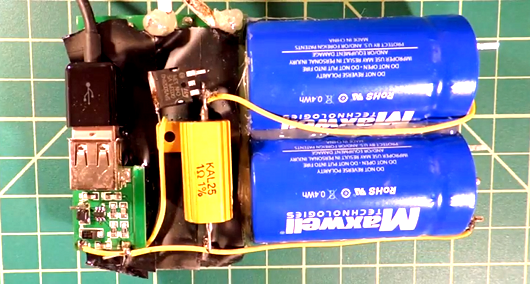
 [Barry] sent us a tip about a video from [electronupdate], describing an experimental cell phone charger. It’s a familiar issue: Your cell phone battery is low, and you aren’t in a position to plug it in for hours to charge. Some phones, including the one in his video, have swappable batteries, but that isn’t always an option either. As he explains in the video, a wall outlet can deliver the joule capacity of a high-end battery in a matter of seconds, but it is impossible to charge a battery that quickly. Capacitors, on the other hand, charge near-instantly.
[Barry] sent us a tip about a video from [electronupdate], describing an experimental cell phone charger. It’s a familiar issue: Your cell phone battery is low, and you aren’t in a position to plug it in for hours to charge. Some phones, including the one in his video, have swappable batteries, but that isn’t always an option either. As he explains in the video, a wall outlet can deliver the joule capacity of a high-end battery in a matter of seconds, but it is impossible to charge a battery that quickly. Capacitors, on the other hand, charge near-instantly.
[electronupdate] decided to look at the possibility of using super capacitors to power a typical usb plug. It would allow you to charge a secondary power supply in a short period of time, and then get on your way, letting your phone charge slowly from the device.
His experiment wasn’t entirely successful, possibly because he used 2.7V capacitors, which required a boost regulator and limited the useful voltage range. We think he might have had better success using 120V capacitors and a switching power supply, but it would be nice to see the various options compared.
Oh, [electronupdate] describes using this circuit as you are rushing to your airplane. We aren’t convinced carrying a couple super capacitors through a TSA checkpoint would be the best idea… YMMV.















Really? 120v Supercaps?
There are none.
I had a 30volt .02 f blow it hissed for a few min. wonder what a 120v super cap would do.
If you know where to get 120V supercaps, I’d love to hear it…
Exactly. there is no such thing. It was even discussed on hd earlier…
http://hackaday.com/2011/04/15/variable-super-capacitor-battery-provides-power-on-the-go/
You could just chain a lot of them together, but otherwise they don’t exist. It deals with the chemistry of the capacitor, the electrolytic that super caps use has very low breakdown voltage.
Also The biggest problem I see here is the discharge curve of the capacitors, you lose a lot of voltage on the capacitors very quickly, and depending on the boost converter being used it is possible that it will fall below useable voltage before you even get a good charge on your phone.
That is why this is an experiment not a commercial product.
“You could just chain a lot of them together”
Sadly, no you can’t. Well, you can, but each cap in series drastically reduces their overall combined capacity, so stacking up 2x10F @ 2.7V caps would yield 5F @ 5.4v. A group of 6 360F caps @ 2.7v would yield only 60F @ 16.2V.
They don’t work like batteries.
chaining capacitors in parallel (not series) sums their capacitance value. It’s when they are in series where you get a value lower than the lowest of the string. The advantage to series is that the dielectric voltage of the caps sum in series. so you could string 6 x 2.7 supercaps up and start your car with it.
https://www.youtube.com/watch?v=z3x_kYq3mHM
I’m pretty sure that’s what I just said. Also, it was that exact vid I was using as my second example.
I know I wouldn’t feel comfortable having portable 120V in my pockets if something went wrong.
Also, do these people have any idea how much less efficient it is to use a 120V to 5V switching adapter vs using a switching regulator from 5.4V to 5V? Geez. Also, just the size of the 110V adapter alone would be offputting. Sorry HAD, you dropped the ball with that suggestion.
I love the idea! I have some 400V 10’000uf caps here that may do the job. Defiantly needs to be a switch mode and not a regulator. As an alternative you could always look at the high charge rate batteries used in the older RC Cars and the likes.
I cant no longer count the times that I head out to another site after a busy day and having used the phone most of the day and not been able to charge it. A portable charging bank would be quite handy especially one that can be charged in seconds.
Energy for each one of those capacitors is 1600 Joule if you charge your cap all the way to 400V . So you only need 6 of them to equal one AA battery. If you charge the caps to 120V, they’re only 144 Joule, so you’d need only about 70 of them.
I have 8 of them :) and to check when they are fully charges apparently you place your tongue across the posts..
But seriously, I suspected it would not be as easy as that, also theses things are BIG and not exactly the king of thing you want to be dragging around.
Boris
First, you’d need a suitcase worth of supercaps to store the energy of a phone battery. The energy density is nothing like as high.
Secondly, although you could charge supercaps up in a few seconds, you’d need a few hundred watts of power supply to charge them up, assuming you were aiming for, say, a 5 minute charge.
If this was any use at all, there’d be no point in having the batteries and manufacturers would just put supercaps in instead.
There’s an alternative to this available very cheaply at pound shops and places like Ebay. It’s a box that takes ordinary batteries and outputs 5v. Takes longer to charge your phone, but is actually capable of doing it. Usually you’d just use enough of the charge to make your calls.
Of course using primary cells is a much more expensive way of charging your battery, which is why those rechargeable power-pack things exist. And they’re heavy and bulky. Which is why you could just get a larger phone battery, third-party or official. Of course, they add weight and bulk too, which is why it’s easiest to just make sure you keep your phone charged.
My solution to this problem was to swap the internal battery of a rayovac charger with an old cell phone battery. I can still charge the battery with the USB port, and it provides enough juice to get about 50% on a dead iPhone. The swapped battery doesn’t get hot, during charge or discharge. I have even used it to top off my kindle fire. It takes a bit to charge, so I charge it and store it as a backup. Since the iPhone doesn’t have an ‘easily’ removed battery. And, really not that bulky either.
Until super capacitors are cheap enough and high amperage wall warts are cheap these experiments will remain experiments, you could buy a small jackery bar for about the same cost maybe a bit more than this circuit but it would fully charge your phone and fit in your pocket
http://www.amazon.com/Jackery-Ultra-Compact-Lipstick-Sized-ThunderBolt-Blackberry/dp/B00AA6CS86/ref=sr_1_4?ie=UTF8&qid=1383478028&sr=8-4&keywords=jackery+bar
I have yet to buy one, just heard of it from a commentor on engadget, will probably get one eventually if I ever find that I kill my phone more and more, but I usually play music on the car stereo over usb so I will likely never have it unless its more than I want it lol
Same idea. But, what’s the fun in picking it up off the shelf.
heres a different take – why not connect many small li-ion cells in parallel, this way it is less efficient in volume, but they can charge faster. needs a more complex regulator too.
Not a suitcase full. If you read the label in the picture, each supercap is 0.4Wh. In a perfect scenario, you’d around 12 of them to charge your average smart phone.
He is baking up the wrong tree, and it’s because he failed to do any of the math before even starting the project. Kids there is a reason you need to learn the maths for electronics, so you can design on paper, and you can even run it on paper (Yes you can) and see if it passes a sniff test before you waste money and time on building a prototype. Or worse, have a good idea but waste money on the wrong parts so that your first working prototype is either 80X what it should have cost or never built because you ran out of money.
Supercaps do not have anywhere near the capacity he wants in the formfactor he is after, It’s like trying to build an ipod nano in 1939. IT just can not be done because the technolgy is not there yet.
Put the idea on a shelf and revisit researching the technology yearly, and one day you will find, “Oh look 2800mah supercaps are available now!” and you now have a viable project.
It won’t be a project because there wouldn’t be any need for batteries, supercaps would be in the phone to start with. Unless they’re REALLY high capacity batteries, and phones come with welders or floodlights.
They could have built an iPod Nano in 1939, sure it would have been the size of the Pentagon, needed the total power output of the Sir Adam Beck Hydroelectric Power Station at Niagara Falls and cost more than the total economic output of the planet at the time but it could have been done, it was just easier to buy a record.
They could have built something that plays music, but it would not be an iPod Nano.
No they wouldn’t because the signal propagation delays on a 1939 valve computer would be so astronomical that it wouldn’t be able to decode an MP3 in real time no matter how. It just simply would not be fast enough.
Maybe they could do stuff like the FFT in discrete components. Or even something like a load of tuning forks. They made up for the primitive technology with massive ingenuity! And they didn’t know how primitive it all was.
The storage would be a bugger. How fast can you read magnetic tape, in bps? I suppose punch tape would be out of the question, unless it was made of inch-thick iron and wound by a rocket engine. Maybe something with spinning mirrors and microfilm?
Just to add, they had vocoders, as an early attempt at voice encryption. in WWII. Wasn’t brilliant, but worked in realtime. And that’s not a million miles from MP3. And racks and racks of equipment was no bother for them. There’s probably a lot you can do in parallel for MP3 decoding.
Of course to produce the MP3s it’s either more tuning forks or roomfuls of women with slide rules.
How about using multiple lower voltage (~6v) supercaps in series during the fast charge up, and then using the caps in parallel with a down converter for charging your phone?
I don’t really know why, but putting capacitors in series reduces their capacitance. If someone wants to explain that to me I’d be grateful. AIUI it makes it a lot less easy to add up caps with lower voltage ratings.
Actually don’t bother explaining it, I’m looking it up on the web. I’ve read it in books going way back and it still hasn’t clicked. Just make a better job of my answer, if you like.
Its because of Magic. Pure Magic.
The best “intuitive” way I’ve ever heard explaining the reciprocal summing thing is:
Start off by assuming you’re dealing with two identical capacitors. By putting two capacitors in series, you’re doubling the distance between the relevant plates. C=ε·A÷d, so doubling the distance halves the capacitance. This extends easily to explain things as long as you assume both capacitors have the same permittivity and cross-sectional area.
It’s not too hard to get from there to 1/Ceffective = 1/C1 + 1/C2.
Probably a stupid question but would charging two same caps in series with twice there voltage fully charge both? If so would it follow that three caps and three times the voltage would work?
Error– that’s really just a very good way to fry *two* or *three* capacitors instead of just one. You have to remember that the current has to go through *all* of the capacitors involved… one at a time, because they’re not all hooked up to the current source. Furthermore, most circuits use capacitors rated for well over what they’re being charged with and discharging, because they tend to fail at even a little bit over their rated voltage, and at two or three times the voltage it’s often rather spectacular and HOT.
Mathematically, Error_user_unknown, you’re right. But for real-world reasons (the capacitance won’t be exactly the same, the ESR won’t be exactly the same) you’re unlikely to get perfect balancing on capacitors in series.
So derate by at least a factor of 150% and you’re probably ok; we once used three 470V capacitors to build a 1kV bank (to drive a pulse laser)
The capacitence is only lowered if you use the capacitors in series. When you switch the capacitors to parellel, you’ll get C1+C2+C3…+Cn. So charge them up in series but use them in parellel.
Surely when you charge them in series though, they won’t fill to their full capacity, since their effective capacity is reduced? Again, I half-understand this, but I think I’m right on that.
Switching between series and parallel is how a lot of voltage converter ICs work, but in that case the caps aren’t there for long-term energy storage.
Sorry, but I don’t think you can dodge the problem like that.
The capacitance halves, the energy stored doubles.
The amount of energy stored in a capacitor = 0.5 * C * V2
The energy goes up by the square of the voltage.
If you have a 2F 2.5v super cap charged to 2.5V, it’s holding 6.25J
If you put two in series, making a 1F capacitor charged to 5V, it’s holding 12.5J
capacitance is directly proportional to the surface area of the conductor plates and inversely proportional to the separation distance between the plates.
The positive plates of capacitors connected in parallel are electronically the same point. It’s as if the positive plates are one continuous positive plate. The same is true of the negative plates. This physically translates to a larger “surface area of conductor plates”
however, in series, it’s as if they are stacked on top of each other, and the current must flow through one, then the next, then the next… etc.. Therefore, electrically at least, the first positive plate and the last negative plate have a large “distance between the plates.”
This is how I’ve always approached them at least…
You, and RJ who gave a similar explanation, I think have finally got it through to me! It’s about the size and distance of the plates, which I knew anyway, but never thought about for more than one capacitor. Brilliant, I get it now, ta!
Just get a spare battery if you’re away from a USB port to recharge.
Lots of work on a “solution” that there really isn’t a problem for.
The “problem” here is there is no time to charge a battery. The “Solution” fixes this by reducing the charge time of the recharge pack from hours (battery) to minutes (capacitors). Its a great idea in theory, however in practice large enough capacitors that can do this job do not exist, let alone the safety ramifications. I would LOVE to have a supercap i could charge in ~5 minutes, plug into my phone, and recharge my phone on-the-go.
For reference, the energy (in Joules) stored in any capacitor = ½CV^2 . Although convenient (& dramatic if all let go at once !), this is really pretty feeble. Even the most humble electrochem system can store orders of magnitude more energy for much the same pacakage. As examples (with cap. discharge & falling battery voltage etc ignored) –
* A 1 Farad cap at 5 V stores ½ x 1 x 5 x 5 = 12.5 Joules
* A single boring 1000mAh AA cell of 1½ V can deliver 1½ A.V.h = 1.5 Watt.hours
* As energy = Power x time then cell energy = 1.5 W x 3600 sec= 5400 Joules
The “ultracapacitors” sold (relatively) cheaply on eBay are 2600F at 2.5V = 8125 joules. This project is definitely doable with the right parts.
Yes but 2 points,
1. I wouldn’t trust most cheap chinese electrolytics as far as I could spit them. For some brands/types capacity is often off by a fair margin and they pop quite easily, even within spec. Would you want a *MUCH bigger version near your phone or you?
2. The size. Those 2700F caps are ENORMOUS!
But if you only have access to a power source for 1 minute, how much charge can you put in an electrochemical solution?
By the way, it looks like this guy has more like 5V, 230F, not 1F. His supercaps say “0.4Wh” on them. Which if they’re rated at 5V would make them 115F.
A high performance Lithium battery can be charged at about 10C. They’re 3.7V. To compare apples with apples, lets make out battery 0.8Wh to compare to this guys two supercaps.
That gives them a capacity of 216mAh. You can only safely charge it at 2.16A (I’ve never seen a battery that small with a 10C charge rate though, 5C maybe…)
A 1 minute charge at 2.16A will give it 0.13Wh of charge. It’ll take about 20 minutes to fully charge, 8 minutes to get it to 80%
The entire point was to build a device that can be charged in a very short time to slowly charging a chemical battery.
I have a little box I picked up for 50 cents at a yard sale. Pop in four AA cells, flip the switch, plug in a USB cable and I have 5 volts to keep a phone running. It can also use NiMH cells so I have a rechargeable battery powered battery charger.
Be very careful if you try to quick charge a lipo battery. If they are charged at a too high current they can easily catch fire.
Interesting idea but seems like it’s not realistic.
I have a 10Ah portable battery pack that I carry around with me. It charges from a microusb cable and has two usb ports. It takes a very long time to charge but it keeps me going when I’m traveling without access to a power point.
I recently bought a 3rd party pack for my Galaxy S4. They give you 2 batteries and an external charger. The batteries last just as long as the original and it only takes 30 seconds to swap them out.
Shame your external 10Ah pack doesn’t have an option to charge from a mains adaptor with a higher current, the batteries can surely take it. Obviously the adaptor would cost money, but putting a barrel socket on the pack wouldn’t add much, with the adaptor as an extra..
I see two different problems people are criticizing mostly one.
Problem 1 as I see is is how to charge your “portable” device fast. (Seconds-Minutes)
Problem 2 is what is a device that can store X amp hours of power in a portable format.
This device targets #1 NOT #2, anything with a battery is limited by the physics of the chemistry in how fast the cell can absorb power. If I had a way to fill a portable power system in a fraction of the time its worth it. Sure this isn’t great but it did reduce the time by a substantial margin. Size of the “brick” aside, it was what about a 1 to 3 ratio, 1 second of wall charge supplied 3 seconds of phone charge. If the size wasn’t too bad then your looking at reducing the charge time from 10 hours to 3.5 or so not ideal but FAR better.
For the naysayer and the critic, look at Mr Edison. I challenge the next critic that thinks he/she can do better to post their hack with better performance and still fix problem 1 not 2.
Since I don’t have a solution for speed, like a majority of posters I use a portable battery, however in my case its multi use. My Asus laptop has one usb port that even when the computer is powered off, supplies regulated power. Far from ideal but since I have my laptop in a majority of situations that leave my phone and other devices needing power, it works very well.
This sort of thing just seems to beg definition by some sort of modified Engineering triangle. Cheap, Fast, Good. Replaced by something like Fast, Portable, Lots of AHs. Pick any two.
If I recall correctly Tesla already had a solution, remove the local portable supply and replace it with essentially a radio receiver, it almost worked.
This is the best post I’ve seen on this thread.
My solution to charge my devices on the go is use an external battery pack. I’m using a RAVPower 10400mAh. It can give over 6 charges to my Galaxy S2 on a full charge base. I charge it overnight and then can keep my phone charged for several days.
http://www.amazon.com/RAVPower-10400mAh-External-Thunderbolt-Incredible/dp/B009V5X1CE/ref=sr_1_1?ie=UTF8&qid=1383536742&sr=8-1&keywords=ravpower+10400
It won’t be long before graphene finishes the R&D phases and goes into products like smartphones. Then before long all batteries that charge in seconds.
“they tend to fail at even a little bit over their rated voltage”
Electrolytics (not supercaps – they’re more like batteries) can be trained to 2 even 3 times their factory trained Voltage, I’ve found.
But I don’t normally do that, especially for any equipment someone else will end up with. Sometimes, in a pinch though, I’ll use an electrolytic beyond it’s printed Voltage.
working examples: 10 V caps in 12 or even 15 V circuits, specially audio amp speaker decoupling. 16 V caps in 24 V circuits, a few 25 V caps in 32 V circuits
two 70 V 10,000uF caps in series for a small switching power supply getting 160 V, three 35 V 60,000uF caps in series for another 160 V supply (it took several days to train those)
I just put a 6.3 V 2,700uF cap across solar cells a few days ago, that go up to 16 V, and have measured ~ 15 V on it so far.
They become leaky when you do this, and can get warm if you try to train them too fast. If the cap gets warm you have to discontinue the training otherwise it may go into thermal runaway and vent.
Once trained they’re fine, the capacitance is slightly lower but they have wide value tolerances anyway (typically – 20 % to + 80 %)
“would charging two same caps in series with twice there voltage fully charge both? If so would it follow that three caps and three times the voltage would work?”
They tend to self-equalize but it’s recommended to add equalization resistors across them due to tolerance issues. Again I’m talking regular electrolytics not supercaps..
For this project I recommend 4 or 5 fast charge Ni-Cads.
To be able to grab enough charge in only a minute with the fast charge Ni-Cads simply use higher AH rated cells, so that their max charge rate isn’t exceeded, yet you get enough of a charge to satisfy your phone’s requirements.
Say you need 2 AH, use 4 AH Ni-Cads (called high capacity cells) so that the charge rate isn’t dangerously high in the time you have. I’ve found there really isn’t a limit on charge rate, the limiting factor is the heat produced from the charging.
I sometimes charge Ni-Cads (when I’m in a hurry) by putting them directly across 12 V and allowing them to draw up to several amps, (which is WAY beyond what’s considered a safe charge rate, even for fast charge cells) until they start to get warm. Good chargers use a 5 degree rise in temp to terminate or reduce the charge rate, and many Ni-Cad packs have thermistors built in for just that purpose.
It’s hard on them, they prefer a nice gentle slow charge, but it’s still far better than any capacitor, super or otherwise.