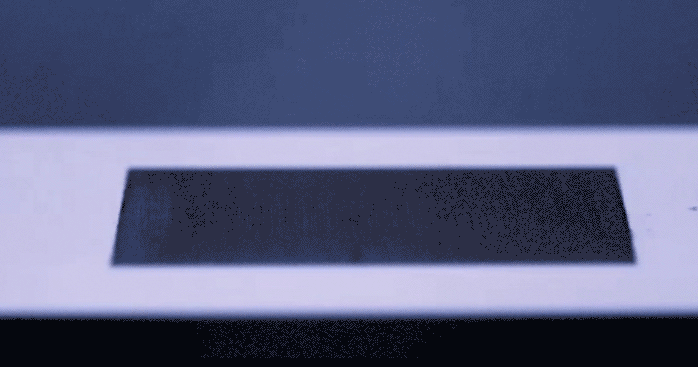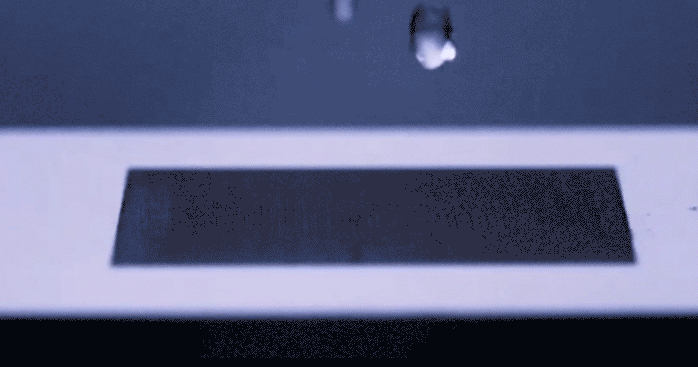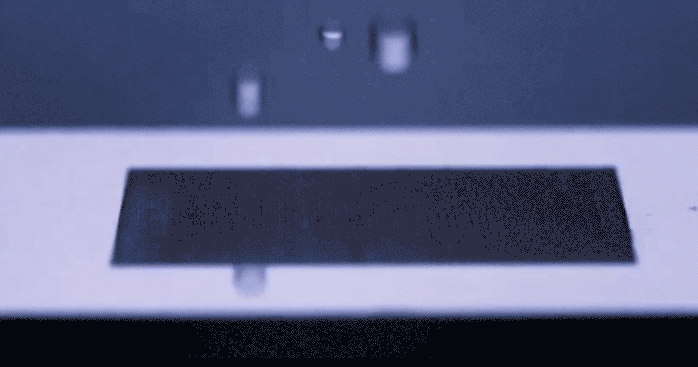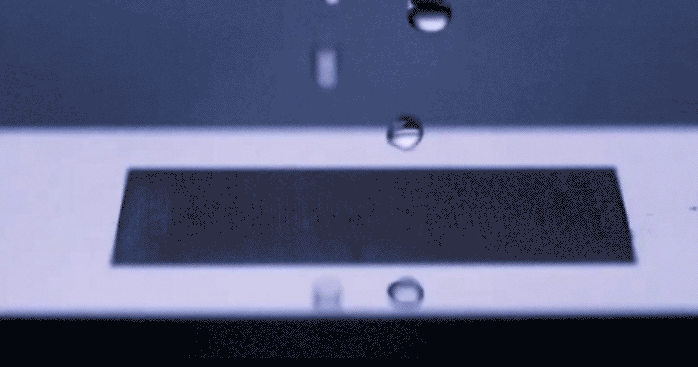
Just the other day we stood in the kitchen making eggs, staring suspiciously at a long scratch carved in the center of the frying pan. With all the articles passing through social media prompting us to be wary of things in our environment that are supposedly killing us, Teflon included, I wondered to myself if humans would ever start coming up with solutions to daily problems… like sticky eggs, which don’t involve the use of complex chemicals. Alas, the universe responds with uncanny timing. A group of researchers led by [Chunlei Guo] from Rochester University’s Institute of Optics has recently published their development of a surface textured by lasers which repels fluid like a rubber ball… without any chemical treating involved. You really need to see this happen in the video below.
This physical magic trick gets its inspiration from nature, mimicking properties of surface tension from living things that repel water such as lotus leaves or butterfly wings. To achieve a similar effect, a precision laser is used to etch nanoscale patterns onto metal which change the surface properties in such a way that fluid molecules prefer not to stick. The benefit to texturizing a material’s surface as opposed to glazing it in some other repellant, is that the pattern becomes intrinsically part of the surface structure and will not fade over time the way a chemical seal will chip or flake. This hydrophobic technology could improve the way we keep surfaces sanitary as well as lend itself to new methods of frost prevention. Not to mention the dozens of other less important applications that we’ve just thought of for our own amusement.
In addition to creating the hydrophobic surface, the Institute of Optic has employed similar tactics to come up with a material capable of absorbing fluid and carrying it upward swiftly against gravity. With the knowledge of physics and the power of lasers combined, we’re glad to see humans coming up with smarter ways to manipulate the world we live in for a more comfortable daily life.














Works great until you accidentally take an abrasive pad to it or scratch it with a utensil a few times.
well.. that would damage a teflon-based coating as well, so I wouldn’t consider it as a disavantage.
Complete guess: this surface is orders of magnitude more delicate than teflon
i would think it’s just as durable as the material that is being etched… since, you know, you’re just dealing with the structural properties of the actual material rather than a coating…
How durable is titanium *at that scale*? For comparison, how durable is the delicate edge on a well-made chef’s knife? How long can you use it before you have to have your knife resharpened? How strong is aluminum? What’s the difference between the frame that supports the weight of your car and the foil you wrap around you baked potato with your bare hands?
In order to get those ultrahydrophobic properties, the material has to be laser-carved to get a surface structure with lots of very fine pins facing up, just like a pin fin heat sink but at a much smaller scale. So even with a very tough material, these tiny pins are extremely fragile
@Dan
With so many lands so close together would it be possible to exert enough force through an abrasive to bend the base material? A chef’s knife may dull but if you stack enough razors you can stand on the edge w/o injury. The real enemy would be, as another poster noted, acid from food changing the geometry. That can be solved with anodizing or some other form of coating.
@Dan J
I can’t seem to remember the phrase I found it by, but there exist some microscopic images of seasoned cast iron cookware, either SEM or maybe even STM. They show a similar structure to the hypothesized lotus leaf pins; instead of pins it is lots of small pits with connected ridges.
My point is, though, that at such a small scale and not exposed to the sideways force that a knife edge experiences (a knife edge isn’t a nano-scale cutting edge on a otherwise flat surface) the peaks aren’t exposed to the same shearing force that results in a knife needing honing every use. Maybe this hypothetical cookware would require silicone spatulas instead of metal ones, but the benefits could be worth it.
> Works great until you accidentally take an abrasive pad to it or scratch it with a utensil a few times.
If it gets damaged, just take your laser and etch it again.
> How durable is titanium *at that scale*?
Titanium is very light and doesn’t affect a scale very much at all.
> For comparison, how durable is the delicate edge on a well-made chef’s knife?
Knives belong to a class of mechanical separation devices known to contain delicate edges.
> How long can you use it before you have to have your knife resharpened?
Skilled welders have been seen welding razor blades together.
> How strong is aluminum?
Aluminum baseball bats can be used for more than just baseball.
> What’s the difference between the frame that supports the weight of your car and the foil you wrap around you baked potato with your bare hands?
Running over a potato with a car is a proven method of producing mashed potatoes.
I hate teflon pans, when I use a pan I want to fry stuff, not boil it on a piece of plastic.
Properly prepared cast iron is pretty much not stick
Until you wash it. Also, PTFE has PFOA, which is being phased out. PTFE isn’t really of concern unless you overheat it.
http://www.cancer.org/cancer/cancercauses/othercarcinogens/athome/teflon-and-perfluorooctanoic-acid–pfoa
I call polymerized oil a plastic, just because it came from a natural source doesn’t exactly mean it’s terribly good for you when it chips off into your food either.
It’s nice that you can renew the coating on a cast iron pan … but lets not pretend it can approach the release properties of teflon. Even the silicone glazes used in professional baking don’t get close.
I call polymerized oil a plastic, just because it came from a natural source doesn’t exactly mean it’s terribly good for you when it chips off into your food either.
It’s nice that you can renew the coating on a cast iron pan … but lets not pretend it can approach the release properties of teflon. Even the silicone glazes used in professional baking don’t get close.
@ Pinky
There are plenty of natural things that will kill you outright. Natural vs Synthetic is the weakest justification for not using plastic. Fluoro-polymers are chemically inert under cooking and certainly digesting conditions. Any flakes of coating that happen to make it into your food will pass through you undigested and unphased. Unless you make a habit of leaving empty skillets on the burner until they’re red hot with your pet canary next to the stove; Nothing bad will happen.
Your fears are a result of poorly written newspaper articles, not actual fact.
This is only marginally as effective as the leaf of the Nasturtium plant, which has been known for centuries to cause water to ball up and roll off of its leaves. I love it when people get all excited about “new” technology when the exact same technology has been extant in nature since before we could speak. LOL. Good job; next time just grow a plant.
I’m pretty sure the scientists _knew_ about the plant, but were looking for practical applications using modern materials…
More like they found a way to do it with lasers, instead of paints, to create that effect. Putting things through laser engravers are far easier than applying chemical coatings.
did you even read it? the introduction itself says “Nature provides many examples of multifunctional properties on a biological surface. 1,2 One of the examples is the water-repelling lotus leaves.”
Not as easy to cook on a plant :-)
Good luck cooking an egg in a plant.
What about eggplant?
http://i.ytimg.com/vi/XnTdopLyvbY/hqdefault.jpg
I’ve done it before. Slice an onion top to bottom (through the axial radius), remove all but the outer three or four outer layers. Crack the egg into the “pot” and set it directly in your campfire. If the fire is small enough, the egg will cook VERY slowly, and the white will firm to a stiff custard-like consistency. Delicious. And, as a bonus, once you remove the burnt outer layer, you can eat the pot. Also pretty tasty.
I have also been told you can use an orange peel to do the same thing, but I have no personal experience using citrus. If you try this, I personally wouldn’t eat the pot, but that is a strictly personal bias.
This is a hack i’d read about!
https://youtu.be/ZzUXHE1Frr4?t=180
Dude, nature has been working on this for 3.5 billion years, research scientists, not so long, cut them some slack.
Okay, I will now ‘grow’ a plant as you have said.
It will be a power plant to run my laser and etch frying pans.
I will then use it to cook some nasturtiums.
diamond coating etched with this pattern :D
If you heat diamond it turns into CO2. Better to etch sapphire.
I read the paper. It is laser etched titanium.
Very interesting idea, however I do not think it would well as a replacement for teflon-based kitchenware. With normal usage, this intrinsic pattern engraved over the kitchen material would accumulate remainings of food, dust, etc.. modifying the material’s structure and therefore losing its intended properties.
Your speculation is appreciated, but you might try Google first next time: http://en.wikipedia.org/wiki/Lotus_effect
These homegrown scientists randomly throwing out wikipedia articles…
Read the article you linked at least: “As this self-cleaning effect is based on the high surface tension of water it does not work with organic solvents. Therefore, the hydrophobicity of a surface is no protection against graffiti.”
Typical kitchen use is very different from “dirt falling onto lotus leaves”. Kitchenware gets a lot of organic stuff with different surface tension boiled and fried on its surface. Then it gets washed by who knows what kind of soap. Plus repeated scratching of the surface will happen. All this may impact the nano pattern and the effectiveness of this effect. They only shown us how it works with clean water (he even said “we want” to make it work with contaminated water), frying an egg on this could be very different.
You might read the abstract. It mentions mimicking the way the lotus leaf and some butterfly wing is structured so that dirt, dust, and other contaminates get washed off by the repelled water. The water does go between the micro- and nano-structures for an instant, picking up the junk left there, before being repelled with enough force to self-clean.
Paint would, apparently, still stick because most spray paints (your graffiti reference) are not water based. The pigment would get inside the micro-structure and dry there. In cooking, this isn’t normally a problem because you would have either water soluble (cleaned by method in abstract) or oil soluble (cleaned with an amphiphilic substance and water). The problem of staining a pan from a natural pigment in the way that spray paint would is mildly absurd, as I don’t think either cast iron or teflon would shed spray paint too well either.
As Quin said, the abstract also touches upon the self-cleaning effect. I’d have linked to it instead, but that seems redundant seeing as how the article on this page (that we’re discussing) already did that.
If you’re going to call people out for speculating, demonstrating that you read the publication would lend to your credibility.
Wouldn’t you just need to rinse with water or maybe a gentle solvent and wipe off with a cloth? Since the water won’t actually stick to it, it seems like a rinse will get everything off. Need to field test….
I feel like water would be bouncing all over the place when you’re trying to wash this thing.
:)
It might not work well with cookware, but there are plenty of other applications. Low-friction plumbing, self-cleaning solar panels, rust-proofing iron, …
Wouldn’t work for iron, you still have water vapor and oxygen around…
That is really neat, but it would be useful to compare with household oils and detergent water solutions of differing concentration.
Indeed, detergents and acidic food would likely blunt and smooth surface features. Might make a great umbrella though. And if the surface remains smooth enough, windows would benefit. More real world testing is needed.
I am betting the durability of this surface is very very low.
And what is the surface made of?
What would it be damaged by?
made of titanium
Regardless of the surface being made from the base metal, it’ll still be prone to wear and scratches; something that would mar the finish. This is especially true when you begin talking about the micro-structures that form the surface. Even if it’s titanium or some other abnormally strong metal, you can still deform it if the cross section is small enough.
All of that said, I’m not saying that their surface is not more durable than say teflon, but it’s certainly not indestructible; even in normal use.
I wonder if they could make thin sheets of tool steel or a carbide and line the pan with it. Just use plastic utensils just like with teflon and it should last a long time.
I wonder if you can make a useable non pressurized water film bearing by using opposing surfaces textured with each process.
I vant ze speedboat made out ov it.
That would be da BOMB!
However, you may want/need a non-hydrophobic stripe above and/or possibly below the waterline. With a hydrophyilic stripe slightly above the waterline, it would grab the water as the boat heeled into the turn, allowing potentially neck snapping maneuverability.
or perhaps the propeller blades?
If the fluid doesn’t wet the surface isn’t heat transfer from the surface to the fluid significantly diminished? Not a good property for cookware.
Damn that’s a good observation!
This is fine with water. What about other chemicals that have less surface tension? Could this be used as some sort of filter? Design the etching that would let certain molecules pass and hold others regardless of there size like most filters do now????
I think they do that already, albeit with less exotic materials. Of course such filters only work on water/oil (oil/water) emulsions, not on actual solutions. Or you can have something like zeolites, which trap individual water molecules within their structure, but they are more like desiccants than actual filters. That said, I don’t know of any filters (unless you count size exclusion chromatography) that would filter out molecules based on their size.
“I don’t know of any filters… that would filter out molecules based on their size.” What about chamois? It can be used to filter water/contaminants out of adulterated gasoline. http://en.wikipedia.org/wiki/Chamois_leather
Way more interesting than cookware is the use of something this hydrophobic in desalination plants. The video indicated that dissolved solids were something they tested against, which would be amazing. Current coatings are fragile and expensive as hell so this could make a big difference.
I want it on condoms.
Um…it’s not “Rochester University,” or “Univercity of Rochester.” The name is “University of Rochester.”
How about a ceramic cooking surface etched with the pattern?
It might be hard enough to avoid the problem of smoothing out the etching after repeated cleanings (assuming it’s a real problem, of course). The pattern might have to be etched before the ceramic is fired, but I guess there’s only one way to find out. Either way I hope it’s not *too* hydrophobic or we might pour a cup of water into a saucepan and watch most of it bounce right back out and end up all over the stove.
And if the etching is fine enough to not reduce optical clarity to a noticeable degree then like was mentioned above it’d be a great option for windows or treating lenses in eyeglasses to keep the rain off (or for that matter any lenses used outdoors such as binoculars, cameras, rifle scopes, etc.).
Camera lenses, wind shields, motorcycle helmet visors would all really benefit if it were clear or could be made clear.
All windows.
Also the inside of glasses and pitcher.
RainX
It is a wonderful engineering achievement and lots of fun to watch. These types of nanostructure surfaces do work well when new and clean. I remain unconvinced that real world applications will work as well as the laboratory demonstration.
Another water repellent product called NeverWet works really well until you get detergent or oil on it. Mild mechanical abrasion also destroys the coating. It is very entertaining to see, but real world applications of such an easily damaged coating are hard to find.
I’d bet that the reason natural hydrophobic materials such as the Lotus plant work well is because the plant regrows the coating as it is damaged.
Cheers.
I’d like to see if it could be used on the leading edges of airplane wings. Their main source of damage would be due to dust and dirt impact, but in general, that should be pretty low. The benefit of not forming rime ice? Pretty darn high, if it keeps the plane from crashing due to icing.
Test the surface with super cooled microscopic droplets of water before building an entire airplane.
I have a question that I have thought about in the past regarding hydrophobic surfaces/coatings: how would two closely mated surfaces (such as in a hydraulic piston) work? Could it be used as a replacement for the pressure rings/packing?
And, what about steam? Would this technology work with well mated surfaces to keep steam in a cylinder?
You’re all wrong! It’s Rabies!!
ooh! Car Windows!!!
Cast IRON! No toxic coatings and it will last indefinitely(1000+ years) if maintained. Properly seasoned cast is non stick. If you need to boil water or make soup use a stainless steel pot.
Don’t use cast on the wife’s glass cook-top that shit will probably scratch, then she will use the cast iron on you!
Love my cast iron frying pans and Dutch ovens. I have a glass top stove and use cast iron on it regularly, secret is to lift and move, not slide. Also keep the bottoms and the glass top clean.
Cast iron truly is great for cooking. WONDERFUL heat transfer (and heat holding), non-stick, and (with practice), can be cast in your back yard.
As far as maintenance, you do have to clean it PROPERLY (no detergents), keep it seasoned, etc; but it’s really no different from any other non-stick.
The best thing about cast iron is that you basically cannot permanently screw it up, unless you just let it rust away (and that in itself would take decades unless you are doing something really bizarre). There are various ways to restore cast iron. I like the big fire method: build a big, hot campfire, heat the item to red heat, and then allow it to cool down. Re-season the whole thing (not just the cooking surface). Yes, this process takes a long time, but to season properly takes three or four hours anyway.
Sandwich 2 surfaces – one hydrophobic, one hydrophilic. Run a home brew [or mash] through this. The surfaces should – in theory – remove the water, leaving a higher alcohol content.
Yes it’s a still [distillation]. Without heating.
If they could make a combination superhydrophobic / vantablack surface it would be great for solar thermal collectors.
Great piece of work. What I don’t get is the OP’s problem with chemicals. “I wondered to myself if humans would ever start coming up with solutions to daily problems… like sticky eggs, which don’t involve the use of complex chemicals”?
What? A human is THE MOST chemically complicated thing we know of! You can’t live without complex chemicals. Lots and lots of different complex chemicals. So many they are not even all identified. I suppose this is a side effect (or the desired effect) of the anti-thinking version of Al Gore Bill Nye green clean Earthday WE are destroying the Earth (but THEY are not) spew in the schools.
Reading too much into it? Maybe :-)
I see a lot of people suggesting that this could hopefully be applied to glass.
Has everyone forgotten that it already has been done?
http://hackaday.com/2012/04/26/coating-technique-makes-glass-you-cant-see/
Their method used photolithography, which, knowing the differences, I have to assume is quicker, eaisier, and cheaper on production scale.
I just had a thought: could you do this to the inside of a mold, and injection-mold a plastic thing with super hydrophobia/hydrophilia? Maybe the hydrophobic one would just resist the efforts of the plastic to take its shape, and the hydrophilic one would suck up the plastic and never release the part, but I think testing is definitely needed.
Gee folks. Why all the preoccupation with cookware…something like four out of five posts? Personally, I like the application, mentioned in the vid, of using it on toilets. Imagine just swishing out the toilet instead of scrubbing. Imagine water-free toilets, like the current flushless urinals, only better. Many, many more applications for this technology than cooking an egg.
You are going to have to make the pipes and sewers and everything out of it, or there will not be enough flow to go. Outhouses for everyone maybe? Think globally, crap locally! Di you hear the one about Chuck Noris?
Although I reject your conclusion, the fact is, I don’t care about the pipes and sewers. I care about the part in my house that I have to keep clean.
I thought about this a few years ago. Some other applications I thought of is air conditioners that never freeze up and a wax free snowboard/skis.
So what if this end-state is achievable through other means? Yes, laser etching may be susceptible to wear, damage, clogging, etc. over time. But what if the same surface could be made intrinsic to the material?
Daydreaming for a moment… Say, for example, that crystals could be grown in such a way that the resulting material showed this same hydrophilic/phobic surface? Perhaps those crystals could be made to repeat that surface pattern throughout their structure, so that if some chipped off, wore off, etc., the new surface would show the same structure. If you grew those “magic crystals” with quartz or sapphire, you could have airplane windshields (mentioned previously) that never need wipers, and any dings from dust or sand in the air would just expose more hydrophobic surface. Grown with aluminum, you could have airplane leading edges (also mentioned previously) that again would be impervious to ice, dust, sand, etc.
I’m nobody’s materials scientist, so the crystal structure idea is probably impossible/unlikely, but these are fun ideas.
Wonder what would happen with a submarine with this
Dear Heinz, please coat the inside of ketchup bottles with that kind of material.
Besides, since it was mentionend in the video -> “water repellent toilets”:
As much as the water bounces back, in case of diarrhea, i wouldn’t use it.
Does the Dept. of Justice know of this blatant instance of hydrophobism? Will Congress act? Will His Highness Obama I issue an executive edict?
if the hydrophilic material utilises capillary action to draw up the water, can they place one end in a lake, and the other in a higher reservoir? the capillary action will draw up the water dumping it in a higher location, then release the water through a turbine and generate electricity. since the water is “pumped” into the reservoir with out added energy won’t this produce “free” electricity?
+1
I wanna know!
That is a very interesting idea, but, from the video, the flow against gravity did not appear to move a large quantity of water, and not especially quickly. I realize that more surface area == larger flow rate. I am no engineer, but I am going to guess that the speed at which you can move water from the lower basin to the higher one would limit the size turbine/generator. You could of course run the turbine intermittently, but I expect there would be a practical limit that would limit the overall size.
But, even if it IS tiny, the “free” energy… I would be interested in where the “free” energy is coming from…
Well you can technically do this with supercritical liquid helium in the infinite waterfall experiment as the fluid exhibits frictionless properties. The caveat is you have to keep it at the low temperature. Therefore the energy to keep the helium at the temperature required will most likely exceed the energy produced.
Water would probably wick faster in between two sheets of hydrophobic material (a surprising amount of water can be wicked uphill when it gets between metal roofing panels that were not properly sealed) so maybe the wicking surface area could be maximized by using a tube filled with pleated pairs of sheets (the smaller the pleats, the larger the total surface area)? I imagine a relatively small pipe could contain a huge amount of wicking surface area this way and it might be enough to “pump” a useful volume of water for some purposes.
If nothing else it might be able to fill an elevated “backup” reservoir that feeds a micro-hydro turbine when needed (if the flow is too slow for constant use).