Ever noticed that a rubber band gets warmer when it’s stretched? The bands also get cooler when allowed to snap back to relaxed length? [Ben Krasnow] noticed, and he built a rubber band cooled refrigerator to demonstrate the concept. The idea of stretching a rubber band to make it hotter, then releasing it to make it cooler seems a bit counter intuitive. Normally when things get smaller (like a gas being compressed) they get hotter. When pressure is released the gas gets cooler. Rubber bands do the exact opposite. Stretching a rubber band makes it hot. Releasing the stretched band causes it to get cooler.
No, the second law of thermodynamics isn’t in jeopardy. The secret is in the molecular structure of rubber bands. The bands are made of long polymer chains. A relaxed rubber band’s chains are a tangled mess. Stretching the band causes the chains to untangle and line up in an orderly fashion. By stretching the band you are decreasing its entropy. The energy of the molecules in the band don’t change, but entropy does. All the work one does to st retch the band has to go somewhere, and that somewhere is heat. This is all an example of entropic force. For a physics model of what’s going on, check out ideal chains. If you’re confused, watch the video. [Ben] does a better job of explaining entropic force visually than we can with text.
retch the band has to go somewhere, and that somewhere is heat. This is all an example of entropic force. For a physics model of what’s going on, check out ideal chains. If you’re confused, watch the video. [Ben] does a better job of explaining entropic force visually than we can with text.
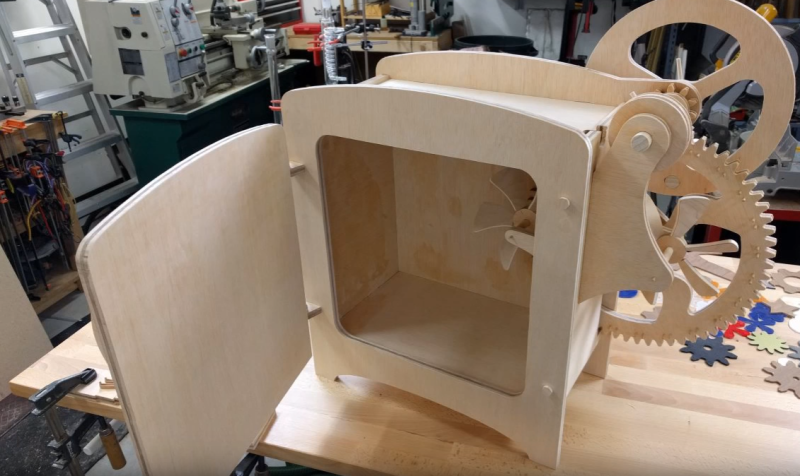
To test this phenomenon out, [Ben] first built a wheel with rubber bands as spokes. Placing the wheel in front of a heater caused it to slowly rotate. [Ben] then reversed the process by building a refrigerator. He modeled his parts in solidworks, then cut parts with his Shaper handheld CNC. The fridge itself consists of an offset wheel of rubber bands. The bands are stretched outside the fridge, and released inside. Two fans help transfer the thermal energy from the bands to the air. The whole thing is hand cranked, so this would make a perfect museum or educational demonstration. Cranking the fridge for 5 minutes did get the air inside a couple of degrees cooler. Rubber is never going to displace standard refrigerants, but this is a great demo of the principles of entropic force.
For more thermodynamic fun, check out [Al Williams] recent article about building a DIY heat pipe.















Wow, never knew they got cooler when relaxed.
That’s why they call it “Chillaxin'”!
Also interesting to think about, and really the same thing when you think about it. If you dip a piece of paper towel in water, the water will wick up into the paper tower. It can climb vertically. We call this capillary action. So… where does the energy come from to lift this small amount of water? Turns out the water cools as it climbs the paper. Super interesting stuff.
Can this be distinguished from cooling effects through evaporation?
I would imagine you could visibly determine this if you had a glass of melting ice, and stuck a thin capillary tube in it, then you’d see it get a short way up and freeze?
that sounds like a very nice experiment, one might have to do it in a temperature controlled room to get enough of a temperature drop, i imagine that the small amount of water in the tube would heat up rather quickly from the surrounding air.
The energy required for a change of state is rather large compared to the specific heat capacity. For instance it takes almost almost as much (about 80%) energy to transition water to ice as it does to cool that same amount of water from 100C to 0C
Maybe you’d want to use a thermometer sleeve on the capillary tube to sit it in the ice water for a bit so the tube is dry but at 0C before you start.
I think you were looking for “Ideal Chains” not “Idea Chains”.
Also, this is a fantastically cool idea for a thermodynamics class project as well. Cool article.
Whoops! Fixed it in the text. Good thing It wasn’t “ideal chins”!
“Rubber bands do the exact opposite.” This doesn’t sound right. It’s relaxing from being stretched which is not the opposite of being compressed.
Same thing. Get bigger, get smaller. Think of a gas already compressed, that’s being expanded, instead. Or don’t, it’s just an analogy, and I think a good one.
This was a neat video, but I found myself wishing he had kept the door shut and had a thermocouple in there to measure the temperature over time while it ran with a motor over a few hours.
Well if the max heat up from the stretch is <= 2 C as he said and he is cooling by air the bands can only be a max of 2 C lower than room temperature which means the fridge would have to be above 2 C colder than the room temperature right?
Yes, I don’t think you’re going to get any temperature difference that’s too exciting, but measuring with a thermal camera can be deceptive. A contact temperature sensor would be better in this case.
“Stretching the band causes the chains to untangle and line up in an orderly fashion.”
One would think that this is also in a way compressing the molecules in the rubber band which then expand when the rubber band is allowed to return to its normal state. Similar to how gases are compressed and allowed to expand again to cool a standard fridge.
I’m thinking of it as constraining the amount the atoms/molecules can rattle, thus forcing energy to be given up.
Don’t try to compress rubber, it will come back with a poisson ratio of 0,5.
(https://en.wikipedia.org/wiki/Poisson%27s_ratio)
Brilliant. More tests please.
A wizard did magic.
Very intriguing!
Wonder if other materials have the same property?
Memory wire has similar properties.
What a great video. And a good demo of the Shaper, too.
mind blown
if one used a strip of Nitinol, the effect would be far far higher in magnitude, worth a try for a demonstration I guess. However as an engineer must point out the the system efficiency is not even close to making a “product” from these methods.
Wouldn’t you have to actually move the individual atoms of the wire back to their origin state? Which is impossible. Just un-bending it wouldn’t work.
i imagine you’d have to make a spring that doesn’t overbend the material so it recovers fully.
Richard Feynman had a wonderful interview in which he talked about this subject. You can see it here https://www.youtube.com/watch?v=baXv_5z7HVY
Awesome video, thanks!
It needs some wax on the shafts so they wouldn’t get so warm where they spin in their supports. Hot ‘bearings’ inside the box you’re trying to cool (however little) defeats the process.
I’m surprised the rubber bands could extract more heat than the fan was putting into the fridge…
I watched the video right till the handheld cnc router appeared. I am starting to get sick from that PR campaign by shapertools. Come on, stop it.
It’s Ben Krasnow – the video will be good, and if he promotes a tool it’s because the tool is good. But, their campaign has been rather extensive so I can see your point.
I did some experiments with rubber bands long before this video came out. Rubber bands can act as a solid state refrigerant, as you stretch them, they heat up. Then you cool them down in their stretched state, removing the heat energy. When you rapidly return them to their relaxed position, the temperature drops below what it started at. Essentially, if you made a rubber bladder inside a box separating two sides (like a partition), you could attach a linear actuator to the center to pull the bladder, heating it. A fan blowing inside the top half of the bladder would help to dissipate the heat, then when the bladder is allowed to relax, it is much colder than before, creating a cold wall inside the box.
This would also work well with focused sunlight. “Perpetual” (until the bands break/fatigue” rotating wheel anyone?