Looking at the ingredient list of some popular processed foods will produce a puzzled look on the typical hacker’s face. Tricalcium phosphate, thiamine mononitrate, zinc proteinate, pyridoxine hydrocloride… just who the hell comes up with these names anyway? It turns out that there is a method to the madness of chemical name structures. Some of them are well known, such as sodium chloride (NaCl) and hydrogen peroxide (H2O2). Others… not so much. In the early years of chemistry, chemical substances were named after their appearance, affects and uses. Baking soda, laughing gas and formic acid (formic is Latin for ant, and responsible for the sting in an ant bite) to name a few. As more and more chemical substances were discovered over time, a more structured naming convention was needed. Today, the above are known as sodium bicarbonate (NaHCO3), nitrous oxide (N2O) and a type of carboxylic acid (R – COOH, think of the “R” as a variable) respectively.
In today’s article, we’re going to talk about this naming structure, so that next time you admire the back of soup can, you won’t look so puzzled. We’ll also cover several common definitions that every novice biohacker should be familiar with as well.
Naming Ionic Compounds
Chemical compounds are formed when two or more atoms stick together. One of the ways they do this is called ionic bonding. It occurs when there is a difference in charge between the atoms, and should be familiar to the hacker as it involves a positive and negative charge. Atoms are usually neutral, with a balance of the number of negatively charged electrons and positively charged protons. However, many can easily become charged by gaining or losing one or more electrons. When this happens, the atom is known as an ion. If it’s positively charged, we call it a cation. If it’s negatively charged, we call it an anion.
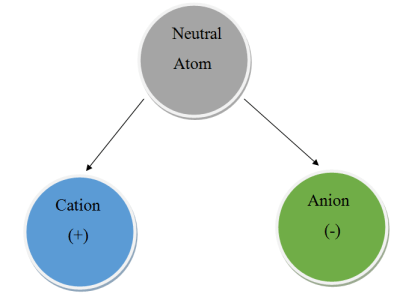
Atoms in the first three groups of the periodic table tend to form cations because the have few valance electrons, making them easy to remove. A sodium cation is simply referred to as a sodium ion. No name change is needed for these groups because their charge will most always be the same as their group number.
It’s a bit different for groups 4 through 7. These atoms tend to form anions because their valence shells are nearly full. They will happily steal an electron to become isoeletronic with their nearest noble gas – the most stable state possible. When an atom becomes an anion, the letters “ide” are appended to the end. Let’s look at an example – sodium chloride.
The sodium atom will lose its single valance electron and the chlorine atom will gladly take it. This makes two ions – a sodium cation and a chlorine anion. Because they now have different charges, they stick together to form an ionic bond. The sodium atom is still called “sodium”, but the chlorine atom becomes “chloride”.
Most ionic compounds are binary. The cation name comes first, followed by the anion name. More examples are sodium bromide (NaBr), calcium fluoride (CaF2), lithium nitride (Li3N)…you get the idea.
Naming Molecular Compounds
With ionic bonding, we were dealing with losing and gaining electrons and forming ions. Atoms can also share electrons with each other. When they do this, we call it covalent bonding. Most covalent bonding occurs between non-metals, and the result is called a molecular compound.
The naming convention is similar to ionic compounds. The first part of the name is the first atom in the formula. The second atom in the formula gets the “ide” added to the end. For example, HCl is called hydrogen chloride.
It is very common for a pair of elements to form different binary compounds via covalent bonds. When this occurs, Greek prefixes are used to denote the number of atoms. Recall that the Greek prefixes are mono (1), di (2), tri (3), tetra (4), penta (5) and so on. So the formula CO2 would be called carbon dioxide. Traditionally, the mono prefix is left off of the first atom if there is only one. But the same rules are in play if there is more than one. For example, N205 would be called dinitrogen pentoxide.
Naming Organic Compounds
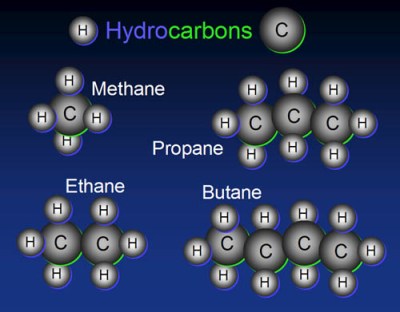
As most of you already know, organic compounds are special. Not surprisingly, they have their own special nomenclature, which we shall cover briefly. Organic compounds are compounds that contain hydrogen and carbon. When the compound is composed solely of hydrogen and carbon, they are called hydrocarbons. The simplest of these are known as alkanes, with their name being dependent on the number of carbon atoms in the molecule.
| Methane | CH4 |
| Ethane | C2H6 |
| Propane | C3H8 |
| Butane | C4H10 |
| Pentane | C5H12 |
| Hexane | C6H14 |
| Heptane | C7H16 |
| Octane | C8H18 |
| Nonane | C9H20 |
| Decane | C10H22 |
Acids
Acids are substances that produce hydrogen ions when dissolved in water. You might have noticed the example of HCl above as named hydrogen chloride. And this is correct. It is only when it is dissolved in water that it becomes hydrochloric acid.
By definition, acids need at least one ionizable hydrogen atom. To name an acid, we remove the “gen” from the hydrogen and replace the “ide” of the secondary element with “ic”. Some examples are hydrofluoric acid (HF, probably the worst acid you’re likely to encounter) and hydrobromic acid (HBr).
This article has only scratched the surface of chemical nomenclature, but should give you a good footing in the subject, and hopefully a look of understanding next time you read the ingredients of your favorite processed food.















Most dangerous chemical out there!
di-hydrogen, mon-oxide
Look it up, I am not kidding!!
It’s brutal, death by inhalation, dry gaseous phase flays your skin off, solid phase can sink a ship, nasty stuff.
Yep, Good substance for serial killers, very useful to submerge the victim in it. Readily available on the daily use…
Away with you…
Not only that but contamination levels are endemic, it has been found in the kitchens and bathrooms of 99.99999% of american families.
How does the .00001% of american families handle the solid waste byproducts then? I can only thing of one likely movie reference about the subject.
“History of the world part 1” Dealing with Roman Pipes. Had to go to the theater or cable to see that scene.
So little is generally known about this compound. That when people where surveyed about it. 95% would support an outright ban of the stuff. I have since had low expectations about the general public.
I could give you a sample of CH4 that would change your mind about that.
Absolutely lethal! 100% of all people who’ve ingested di-hydrogen mono-oxide will die.
pyrodoxine hydrochloride check your spellling it sucks
lol so does myn
” “Disodium guanylate”. That would make a great alien name, don’t you think?” Vala Mal Doran, Stargate SG1
If you’re gonna write a technical article on the proper nomenclature USE FRIGGIN SUBSCRIPTS. Us peasants down in the comments don’t have access to them, but as authors there’s no excuse for you not to use them.
It’s fine in a broad primer that you don’t want to get into acid-base chemistry but an acknowledgement that Arrhenious’ definition isn’t the only used by chemists would go a long way in preventing future confusion. There’s also an implication in your writing that acids only have 2 elements in them and it’s always a halogen.
Historically it was thought, acids had to contain oxygen – hence the german name for it “Sauerstoff”. “sauer” means acidic” and acid is called “Säure”.
Is the O in dinitrogen pentoxide really a zero ?
What w0uld there be to gain in that?
Maybe their ‘o’ key is br0ken, 0r it’s a sign for the illuminati, or maybe the article was written under duress?
Will. If y0u can read this we can help y0u. Just give us a an0ther sign!
Always worth a reference. (only 16 years ago…)
http://megatokyo.com/strip/9
Relax, we understand J00
In any other context I’d agree but chemistry has numbers and letters mixed together out of necessity L337 S|>34|< makes complex formulae indecipherable. A misinterpretation can give a completely different chemical. Even just the simple act of writing the string of letters in reverse order can give you a different chemical. -SCN vs -NCS is one that springs to mind.
It’s leet-speak for N2O5.
The organic compounds get a lot more complicated once you start introducing double and triple bonds. The same prefixes are used when there’s one double bond: ethylene, propylene, etc; and with a triple bond it’s proylyne (the two-carbon version is acetylene). Lots of other rules when you add other bits in there: an oxygen in the middle is an ester, an -OH on the end is an alcohol… go visit here: http://www.chem.uiuc.edu/GenChemReferences/nomenclature_rules.html
I’ve never heard of proylyne, nor has google so I assume you meant propylyne, which is not a compound given the distinct lack of electrons in the proposed system. You’re thinking of Propylene, which would have a double bond and is similar in heat output to the discontinued blend known as MAPP/Razor gas. Acetylene is better discussed in this context as Ethyne so as to avoid confusion in naming conventions vs historical names.
Also while the prefixes in simple hydrocarbons remain the same, the suffixes do not, -ane, -ene (as in keen), -yne (as in mine), for single, double, triple bonds respectively.
I think he means propyne, which would be the three-carbon homologue of acetylene (though it’d rearrange to the allene quite readily I imagine).
Yeah, it does get confusing when you bring in trivial/historic names vs. systematic names. If you mentioned ‘ethanoic acid’ or ‘ethyne’ to most chemists though I think you’d get funny stares. The trivial names in my experience are in much greater use than the systematic, which are generally used for more complex compounds or on paper as part of published characterisation.
The old term “Liver of Sulfur” is still used to describe a poorly defined mixture of various sulfides and sulfates. The stuff is used in the metal finishing trade to create a conversion coating generally referred to as antiquing on copper and its alloys. I know of no other industrial chemical still sold under its ‘alchemic’ name (as it were).
Lots of chemicals still can be found under their alchemical names. These names are very common with people who do precious metals refining. Many others just never got changed to anything else because there was no reason to.
Aqua regia, caustic soda, caustic potash, flowers of sulphur, litharge, lye, muriatic acid, quicklime, sal ammoniac, verdigris, and very occasionally oil of vitriol.
A handful more are ores of certain sulfides compounds (cinnebar, stibnite, &c) and it wouldn’t make sense to change their names after discovery and classification.
Sure there are, but none of them sold exclusively under those names, nor are those names used too often in modern chemical formularies – Liver of Sulfur is almost always called out as such.
Flowers of sulfur, lye, the various potashes, quicklime, and muriatic acid are very much used in commerce. You can get a whole block of sal ammoniac on amazon right now for use with soldering irons. And verdigris at a variety of art supply houses.
Sure your Sigma Aldrich & Acros sales rep might look over their glasses at you but there are many technical supply houses that market items under these archaic names.
Perhaps you missed my use of the term”exclusive”? Yes these names are still in general use but as you wrote, they do cause lifted eyebrows, as does a inquiry about hepar sulfuris, that few know is the technical name for liver of sulfur. And that’s the point I’m trying to make – it is just about the only bulk chemical still commonly called by its old name almost exclusively.
Let’s not forget cream of tartar is the only commercial name I have seen for potassium hydrogen tartarate. The proper name would never fit on those small spice jars.
That’s true too
I see anion and cation but where is the ration?
Formed at the rathode of course.
They are only given out on a as-needed basis.
A+
I see you’ve toured the sewers of Paris recently……RAT-ion
As opposed to a rat-iron, which is usually a spare 9 iron, or a poker, in some cases maybe an actual clothes iron if that’s what the missus is holding when she sees it.
since this is an article about chemistry education, i felt the need to point out an error in the article header image.
that CH on the right should be a CH2.
i’ve fixed it and also added a stylistic change (H2N more clearly depicts that N is bonding to the C on its right than NH2 does):
https://i.imgur.com/OzdXL4e.jpg
right, so formic means ant… does that mean that formic acid is a good antacid? ,
“This article has only scratched the surface of chemical nomenclature”
VERY True! Therefore HaD, I relegate this post to the “Click-Bait” bin :-(