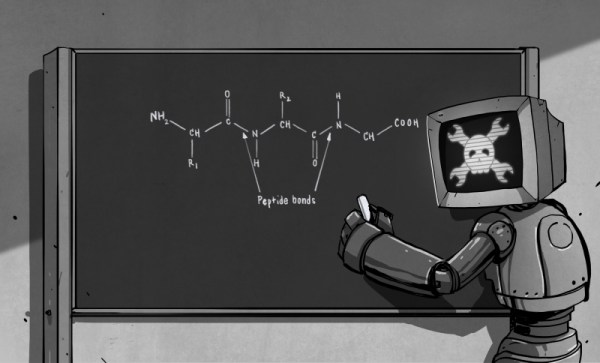
Looking at the ingredient list of some popular processed foods will produce a puzzled look on the typical hacker’s face. Tricalcium phosphate, thiamine mononitrate, zinc proteinate, pyridoxine hydrocloride… just who the hell comes up with these names anyway? It turns out that there is a method to the madness of chemical name structures. Some of them are well known, such as sodium chloride (NaCl) and hydrogen peroxide (H2O2). Others… not so much. In the early years of chemistry, chemical substances were named after their appearance, affects and uses. Baking soda, laughing gas and formic acid (formic is Latin for ant, and responsible for the sting in an ant bite) to name a few. As more and more chemical substances were discovered over time, a more structured naming convention was needed. Today, the above are known as sodium bicarbonate (NaHCO3), nitrous oxide (N2O) and a type of carboxylic acid (R – COOH, think of the “R” as a variable) respectively.
In today’s article, we’re going to talk about this naming structure, so that next time you admire the back of soup can, you won’t look so puzzled. We’ll also cover several common definitions that every novice biohacker should be familiar with as well.