When deadlines loom and your future is on the line, do what top college students through the ages have always done: procrastinate! [Simen] and [Amund] did that in grand style by starting a YouTube channel, delightfully and aptly named “Applied Procrastination”, wherein they plan to avoid their responsibilities as long as possible in favor of making a large-scale ferrofluidic display panel. (Video, embedded below.)
We suppose we should encourage them to hit the books, but honestly they look like they’re having much more fun and learning more than they would in class. The idea isn’t new; we’ve seen ferrofluid clocks before, after all. [Amund] and [Simen] have grander plans for their display, but they’re wisely starting small with basic experiments. They had an early great idea to use a double-pane window as a tank for their display, but coatings on the inside of the glass and the aluminum frame conspired to cloud the display. They also did some tests to make sure they can control 252 electromagnets safely. They did manage to get a small test display working, but really the bulk of the video is just them playing with magnets and ferrofluid. And again, we’re OK with that.
It looks like this is going to be an interesting project, with hopefully regular updates to the channel now that summer break is upon us. Unless they find something else to do, of course.

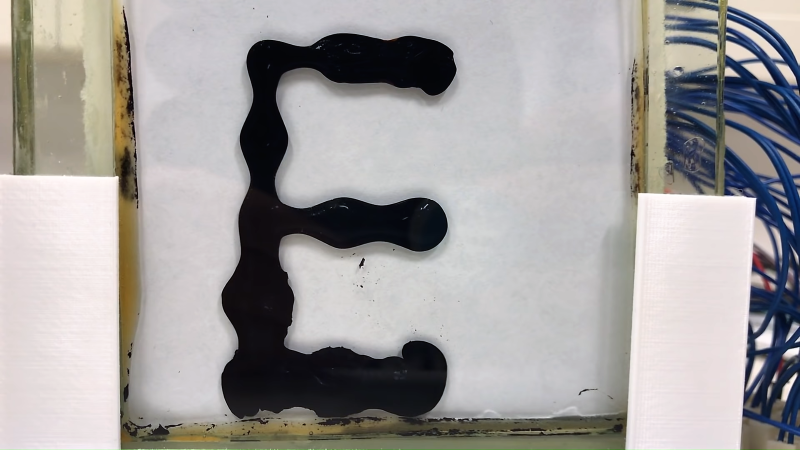











thst reminds me of the game “Badlands” for Android.
The World of Goo?
Cool!
” The also did some tes […]”
” The did manage to get a […]”
Some ‘y’ missing I guess.
Nice project by the way.
FTFY. Thanks
I wonder if they would add a Barbapapa-mode. Considering Barbapapa is a shapeshifter and this fluid sorta does the same if controlled appropriately. For more info about Barabapapa: https://www.barbapapa.com/the-barbapa-family-en
Although I imagine that that would require pink ferrofluid of which I doubt it does exist, so in the mean while they should experiment with a Barbamama-mode which basically is the same except for the colour.
Last I saw on ferrofluids was that they degrade relativity quickly, with the magnetic particles separating from the fluid part. Anybody know how long to expect a ferrofluid to stay stable these days? Would love to do a project with this stuff but wouldn’t be happy if I had to keep regularly replacing the fluid.
Not sure, but that fluid at the start of the video really seized up, didn’t it?
Nile Red is working on a ferro fluid video right now. Check him out on YouTube, he does very solid chemistry videos, and I’m sure thus next one will answer questions about the junk.
I’ll be interested in that as well. I’ve tried to make ferro fluid numerous times, but my biggest issue is keeping it from sticking to the wall of whatever container I put it in. You either need an oilophobic (sp???) coating, or a clear fluid that attracted to the container more than the oil based ferro fluid. I’ve gotten about a minute before my ferro fluid ends up stuck and smeared all over the container.
I’ve had good luck placing it in a glass container with vodka.
I’m a subscriber of Nile Red though I haven’t checked his channel lately, thanks for the tip.
If the ferrofluid were green and florescent it would make a great Halloween display.
I wonder if adding a zinc sulfide or (better-yet) a strontium aluminate based compound could make it glow in the dark (or at least under a UV lamp).