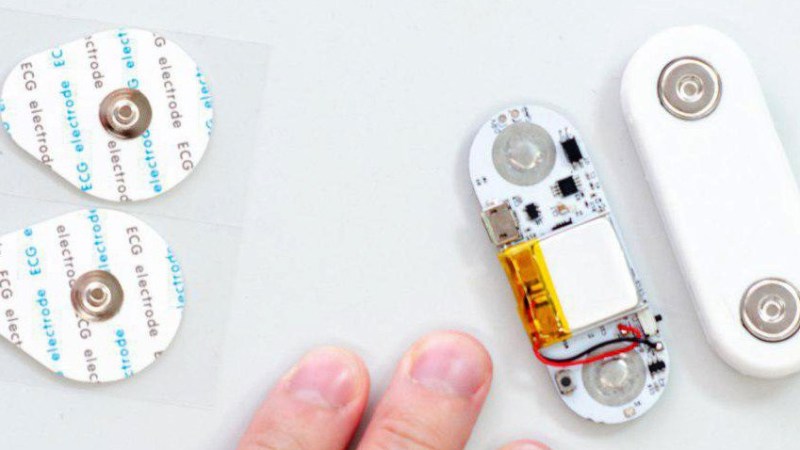
[Ultimate Robotics] has been working on designing and producing an extremely small ECG that can stream data real time.
Typical electrocardiogram equipment is bulky: miniaturization doesn’t do much for a hospital where optimizations tend to lean towards, durability, longevity, and ease of use. Usually a bunch of leads are strung between a conductive pad and an analog front end and display which interprets the data; very clearly identifying the patient as a subject for measurement.
uECG puts all this in a finger sized package. It’s no surprise that this got our attention at Maker Faire Rome and that they’re one of the Hackaday Prize Finalists. The battery, micro controller, and sampling circuitry are all nearly packed onto the board. The user has the option of streaming through BLE at 125 Hz or using a radio transceiver for 1 kHz of data. Even transmitting at these sample rates and filtering the signal of unwanted noise the device draws less than 10 mA.
The files to make the device are all on their page. Though they are planning to produce the boards in a small run which should be the best way to acquire one and start experimenting with this interesting data.

















Ok, an interesting way to reveal pulse rate, however, there’s very little else that can be gleaned from the data this device provides. It “might” be able to reveal A-Fib, though the filtering on the signal may make even that a challenge. Also, “real” ECG systems are polar in nature. Reversing leads reverses polarity and thus the displayed signal. I have seen situations where reversed leads have caused staff to misinterpret the trace. With standard leads reversed, the trace can be misinterpreted as a myocardial infarct (heart attack).
A basic rule in patient care is: Treat the patient, not the machine… So, aside from A-Fib, what might be discerned? Perhaps heart block? but only if the P-Q interval could be discerned and evaluated over time.
Ultimately, from a “diagnostic” point of view, this device really only reveals heart rate and if the firmware is evaluating over an extended interval, regular or irregular rhythm, and perhaps 1st degree or 2nd degree heartblock. So, more information than a pulse-oximeter, but not much more.
It might be an interesting experiment to compare simultaneous traces from this device and a 12-lead ECG. (BTW – if you count the physical “leads” on a 12-lead machine, you’ll only find ten…) :)
As to why 10 and not 12, take a look here: https://ecgwaves.com/ecg-topic/ekg-ecg-leads-electrodes-systems-limb-chest-precordial/
The main point of the device is that it’s open in all aspects – open data protocol, open source app, open source firmware, open hardware design. So if you want something specific from it, you can make it yourself – data quality is good for any analysis (good enough to send out raw data, with only 50/60Hz filtering – not smoothed-out something, like some specialized cardio frontends do).
It is not a consumer product though. Maybe one day it will become one – but not today :)
Just as a note: To make any diagnostic or health claims, a device of this type generally requires regulatory approval or clearance in most countries. In the US, it carries a Class II (moderate) risk classification, meaning that a 510(k) application for clearance is needed. This is a relatively simple task compared to, say, putting a new drug on the market, but still significant effort.
Similar processes in Europe require filing information with a Notified Body who will issue a CE Mark.
This also requires a certain level of quality control of both the manufacture and the documentation.
Other countries require different application processes (China’s is particularly stringent).
I’m not at all saying that this isn’t a fun, interesting project, but if people are going to bet their lives on the monitoring it provides, you’d better have your paperwork in order.
A notified body does not ‘issue’ a CE mark. The CE mark has no meaning other than to indicate that the manufacturer or her rep has written a declaration of conformity.
Many appliances and other common electrical equipment manufactured in the EU, and having a CE mark, would not be compliant for use in a North American workplace. How do I know? I test stuff and review test reports and do tear downs for my clients and employers. To date, I have tested over 200 products from the EU, and less than half were fully compliant, and approximately on third were not safe to use in an office environment.
I have no idea why everybody thinks the EU is such a perfectly safe paradise.
You have to remembre that loads of products are not CE marker as in “it follows EU standards” but as in “I’m a Chinese Export manufacturer making a look alike logo”
But if it can pass that and doesn’t interfere with other medical equipment, and be made and sold for $100, then in the U.S. it can be sold to hospitals for $1,000, and they sell it for Pt. use for $5,000, plus $1,000 a month monitoring at a six month miniumum, with a $200 battery change fee, and the insurance company doubles each fee to get the amount they debit towards the client servicing limits.
I’m assuming your comment is more or less tongue-in-cheek. From a medical/diagnostic point of view, this is not a useful device.
When a hospital tells you you’re getting a 25% no insurance discount, you know a large chunk of why medical care costs so much in the USA.
It definitely could pass FDA if even Apple Watch got it :) Our signal is way more useful (I actually wonder why people assume it’s not – it’s really hard to mess up an ECG device these days, even if you make several mistakes). But it never would – our goal is to make something that people can use on their own, not something to rip money off the healthcare system
To the bottom of the sea with all lawyers.God I can’t wait till we get the first open source MRI machine. I promise to help fund it. I promise to donate my time and resources to engineer it. I can’t wait for the day that radiologist are replaced with software as the first level screeners.I hate the medical industry regulations on stupid things like this. Don’t even get me started on hearing aids.Folks this is an oscilloscope, And by today’s standards are really crappy one. (No disrespect to the designer I love it) let’s not get too legally- excited about it. Any diagnostic information is better than none at all. The monopoly the Medical electronics companies have on medical equipment in this country is straight up criminal. There is no reason I should not be able to go to CVS pharmacy down the street And for $20 get an MRI every six months to be used differentially for screening on things like cancer or other small growths that could be caught early. It’s because of this medical monopoly in the lawyers involved that we have not advanced to this level of diagnostic information yet.
Bravo to the designer! Now it’s time for the Community to start working on open source sonogram machines. The advanced class can work on the MRI. This is a wonderful trend and I can’t wait to see it keep going, Directly in the face of the medical electronics monopoly.
“I can’t wait for the day that radiologist are replaced with software as the first level screeners.”
This is already happening for some forms of cancer (e.g. automatic detection of lung nodules) and plenty of people are working on supporting more pathologies.
“There is no reason I should not be able to go to CVS pharmacy down the street And for $20 get an MRI every six months to be used differentially for screening on things like cancer or other small growths that could be caught early.”
I agree in principles but in practice I would be scared to do a full MRI every six months: claustrophobia + strong EM-fields phobia.
Sounds like there’s room for Innovacion in the MRI machine then isn’t there? If only we had a community full of innovative smart people willing to take chances and do new things… Oh we do!
The stumbling block for an open MRI is not so much the processing for the sense coil data, or the hardware (former & winding) and signal amplifiers for the sense coils.
It’s the enormous cryogenic primary magnet that is rather a roadblock. Few will be willing to embark on a DIY MRI project whose Step (1) is to purchase an MRI machine.
Interesting project. Just a few notes of when I came across designs of similar devices. Please take a look at failure modes, these devices can be quite dangerous if not done right. Please add this information (FMEA) to your public documentation, lets have a discussion on the safety. (It’s not the user that needs to prove it’s unsafe. The manufacturer should prove it’s safe.)
It has been a while so I’m likely wrong here: Take a look at the rules and regulations on these devices. Medical devices are not necessarily diagnostic*. What I found was that a medical ECG will have to survive an AED.
*A medical professional is allowed to base treatment on the readings of a diagnostic device.
Just found out this post exists :)
Thanks for the advice! We won’t mark it as a medical device for a while, not this year definitely, for various reasons. I’m not sure it can survive AED (it’s possible, there are several layers of protection, but never tested, nor designed for it) – but from any practical point of view it’s not needed (AED has more reliable in-built ECG functions, and after applying person would be in a hospital)
Brilliant, I’m interested in this one ins a big way & in conjunction with lucid dream triggers and few other contributory permutations such as minerals, bio active compounds etc etc So having a reliable ECG with post processing connecting probability of causal issues could lead to major health advancements as well as improved sense of well being, great stuff. Thanks for posting :-)