Batteries were once heavy, awkward things, delivering only a limp amount of current for their size and weight. Thankfully, over time, technology has improved, and in 2020, we’re blessed with capable, high-power lithium polymer batteries that can provide all the power your mobile project could possibly need. There are some considerations one must make in their use however, so read on for a primer on how to properly use LiPos in your project!
So Many Types!
With the first commercial lithium-ion battery entering the market in 1991, the (nearly) 30 years since have seen rapid development. This has led to a proliferation of different technologies and types of battery, depending on construction and materials used. In order to treat your batteries properly, it’s important to know what you’ve got, so paying attention to this is critical.

Lithium-ion, or Li-ion typically refers to the overarching technology of rechargeable lithium batteries, but also specifically refers to the traditional cells built in cylindrical metal bodies. The venerable 18650 is one such cell, but a large variety of sizes and types exist. Their stout casings make these cells popular for rough-and-tumble vehicle use.
Lithium-Polymer, or Li-Po refers to a lithium-ion battery that uses a polymer electrolyte instead of a liquid electrolyte. This enables the construction of pouch cells with different geometries. This flexibility of design makes lithium-polymer batteries useful in applications like smartphones and tablets, where a high-capacity battery is needed and a flat form factor is desirable. They’re also commonly used in radio-control models, where their lightweight construction is a huge benefit for flying vehicles.

Lithium-HV, or High Voltage Lithium are lithium polymer batteries that use a special silicon-graphene additive on the positive terminal, which resists damage at higher voltages. When charged above 4.2V, most lithium batteries exhibit significant capacity loss and reduced lifespan. However, by using this additive, cells can be charged to 4.35V without exhibiting these negative effects. This extra voltage provides up to a 10% gain in energy density over conventional lithium polymer batteries.
Lithium-Iron-Phosphate, or LiFePO4 batteries are an altered lithium-ion chemistry, which offers the benefits of withstanding more charge/discharge cycles, while losing some energy density in the tradeoff. They operate ideally between 3.0V-3.65V, instead of the more typical 3.0-4.2V range of a standard lithium-ion chemistry. This, combined with a very flat discharge voltage curve, makes them ideal replacements for 12V lead-acid batteries in many applications, where four cells substitute for the original six. They’re generally more stable, with lower rates of self-discharge and capacity loss over time.
Respect The Limits

Moreso than most battery types, lithium cells are not tolerant of mistreatment. Discharging cells below their low voltage limit leads to the formation of copper dendrites, which can reduce cell capacity or short circuit them entirely. Overcharging cells causes damage to the anode by lithium plating out of solution, creating lithium dendrites, often leading to a short circuit or full thermal runaway of the battery, leading to a release of smoke and flames. Each cell in a pack must also be kept at the same voltage as its neighbors, to avoid cells getting damaged prematurely.
It’s important not to charge lithium cells too quickly. Ambient temperatures also play a big role in battery performance. Lithium batteries don’t appreciate being taken down below freezing, particularly when they’re already fully charged. Below 0°C, charging is impractical, as metallic lithium can electroplate at the negative electrode, causing major damage or even short circuiting the cell. Between 0-5°C, charging is possible, but must be done slowly. Damage will tend to occur when batteries are charged at temperatures above 45°C, too.
Working outside these parameters will quickly lead to a dead battery at best, or a fire and explosion at worst. They also tend to swell up, outgas, and just generally become unseemly to deal with. On the surface this can seem like a lot to deal with. Thankfully the battery-electronics complex has worked hard to solve these issues. With the proper hardware and precautions, it’s possible to use lithium batteries safely and effectively. But anyone working with these chemistries should familiarize themselves with the hazards. Bob Baddeley published a great article on Li-Ion safety back in November.
Battery Tending
For applications working with bare cells or packs, such as when using LiPo batteries in RC models, simply using a lithium-ready charger is enough. The balance leads should be hooked up during charging, particularly when the battery has been taken to a fully-discharged state in use. Ensuring that a smart charger is used with the correct voltage limits (particularly when using LiFePO4 and HV packs) will make sure you get the most out of your batteries. Make sure you’ve got some method to stop discharging the batteries when voltage gets low, whether by a warning light, buzzer, or automatic shutdown.
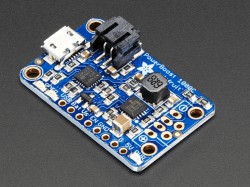
If you’re producing a device that needs a permanently integrated battery, protection and charging circuits are just the ticket. Off-the-shelf modules and ICs exist to take the hassle out of managing a lithium-ion battery. A wide variety are available, from those that act as a simple low-voltage cutoff to complete charging and protection solutions. Companies like Adafruit sell modules that are a great starting point for those eager to integrate a neat charge and battery solution without having to spin up PCBs themselves. However, since these designs are open source it will be easy to integrate the circuit design into your own PCB in the future.
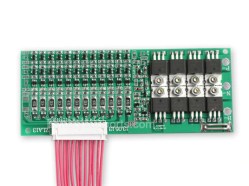
For larger applications featuring custom-built battery packs, a battery management system is a good choice. Basically, a BMS is not much different from a battery protection IC or similar, simply being designed for larger applications. A BMS is typically used on packs of 10 cells and up, used in transport applications like electric bikes and other rideables. The BMS is soldered directly to the battery pack, including a connection to each individual cell. Its purpose is keeping the cells balanced, limiting the maximum discharge current for safety reasons, and of course controlling the recharging process. Experienced pack builders will often integrate a BMS inside the battery’s housing or covering, leaving simply a discharge port and a charge port accessible. This allows the end user to easily drop a battery into a project vehicle without having to worry about handling protection themselves.
If your application is particularly critical and needs to withstand environmental extremes, you’ll want to monitor battery temperature. Keeping an eye on cell temps, particularly during the charge process, is a great way to protect your battery against damage. High-feature protection chips and battery management systems have provisions to monitor pack temperatures in order to achieve this. At this level, you’ll likely be building custom packs, thus allowing you to install thermocouples at precise locations during the build. For high-power installations, temperature management is mandatory, with virtually all e-bikes and electric cars containing hardware to monitor battery temperatures and control systems accordingly.
In Summary
Lithium-ion batteries can bite, but used properly, they offer great performance and are more than safe enough for most applications. The key is to use the correct hardware, to make sure you’re avoiding crossing voltage and temperature limits that can lead to disaster. Hopefully, this guide serves you well as you seek to integrate lithium power into your own projects. And, in the unlikely event you do have an amusing battery mishap, be sure to do a diagnosis and hit up the tipsline. Happy hacking!
















“Batteries were once heavy, awkward things, delivering only a limp amount of current for their size and weight.”
And then there’s lead-acid.
“This, combined with a very flat discharge voltage curve, makes them ideal replacements for 12V lead-acid batteries in many applications, where four cells substitute for the original six. They’re generally more stable, with lower rates of self-discharge and capacity loss over time.”
Perfect for UPS. ;-)
For UPS they’ve got to like being held on trickle charge all the while. The size and weight aren’t really that much of a disadvantage for lead acid in that use, unless you have to fit it in a tupperware in your toilet tank, because tiny house.
I liked the flywheel UPS/Surge Suppressor that IBM used to use on some of their old mainframes. It was basically a flywheel and a genset all rolled into one. Power died the flywheel kept turning the generator and a clutch closed and the flywheel started the backup generator. You show me a surge that can get through a heavy flywheel :)
If ever I see a pit wheel from a closed down mine for sale cheap, I’m tempted to install it under the house (horizontally) for power smoothing and backup.
Windage losses can get expensive. Used to look after some MWh scale flywheels. Their average power input was about 3MW. Great if you want to store energy for short bursts, bad for the long run. Burnt about £2.5M per year on power. Batteries might be a better plan even if a bit explody.
The JET project (experimental fusion tokamak) just south of Oxford has two 775 ton flywheel generators which are used to provide extra power during experiments
Yep, those were the flywheels. Awesome fun crawling all over them.
I’ve seen them time to time in critical applications such as bank data centres. An additional wrinkle sometimes used is a significant reactor between the grid and the protected demand. The excitation on the generator is then used to control the reactive power, and hence the voltage, while the reactor stops reactive power being fed into faults on the grid – mitigating voltage dips.
The trickle charge is you keeping a slight over-potential to stuff in current against the battery’s self-discharge. The fully charged cell voltage is slightly higher than required to break the water to hydrogen (2.01V) so the battery would partially discharge by itself.
With lithium cells, you just keep the voltage up where you want it, because the battery doesn’t leak until it’s way above safe voltages.
That’s also the reason why you don’t need BMS for lead-acid. You just stuff in current until all the cells are bubbling away, so they’re all at the same voltage.
Hey that pic of the BMS, that exact circuit board is the one inside of my kids Gotrax electric scooter! I was -very- disappointed to find out (as I was attempting top fix the battery pack) that it did NOT include a battery balancing charger, so it was just a matter of time until the 18650s (wired as a set of 10 in series) eventually “attacked” their weakest sibling.
Yeah. Balancing chargers are actually insanely rare, way rarer than they reasonably should be. All those crappy app scooters on the sidewalk? I’ve torn down lots of those, and only one of them seems to have a balancer. That one was very strange; it was about ten times better-built than every single other one I’ve seen. I wonder where it came from and why it had so much more effort involved in development. Maybe an early prototype, before they learned to cut costs? Hmm.
So do you just grab the abandon scooters that are on the side of the sidewalk? I’ve been wondering why I have not been able to get a hold of any of those. It seems like scrap scooters should be ubiquitous
“And then there’s lead-acid” – Invented in 1859, I doubt any other useful battery chemistries came before that. So, “and then there’s lead acid” is a bit backward! It was lead acid, “and then” all that other stuff.
Well there was the Daniell cell for the early days of telegraphy.
https://en.wikipedia.org/wiki/Daniell_cell
All this higher and higher energy densities, makes me wonder how much this could potentially be abused. Like for comparison and I know this is going to sound totally crazy (but the maths is not difficult):
The charge that travels through a lightning bolt is typically around 15 Coulomb (for large bolts this can be up to 350)
The charge that travels through a typical alkaline AA battery from being fully charged to discharged is 5000 Coulomb (1400mAh).
( ref: https://en.wikipedia.org/wiki/Coulomb#In_everyday_terms )
It is all a matter of resistance and timing, like the charge travels through a lightning bolt in under 0.2 seconds, where as a AA battery takes a lot longer to discharged by comparison due to its internal resistance.
It is truly astounding how much energy is actually stored inside even a button cell battery, it could be anywhere from 540C (150mAh ; manganese dioxide) and 2232C (620mAh ; Zinc-Air).
You start thinking that the internal resistance of batteries could actually a good thing.
There’s a bit of science fiction that pointed out that there’s little difference between a powerful tool and a powerful weapon.
This comparison breaks when we bring up the insane voltages associated with lightning. Energy is really the fun thing, or energy density, but still. Charge is not energy… volts * charge is energy. (A=C/s, C=As, W=VA, J=Ws, J=VAs, J=VC.) How many joules are in an alkaline? 1.4Ah and let’s pretend for now that the average voltage over its discharge cycle is 1.2 (varies with discharge rate)… so 6kJ, hypothetically (someone says 2.5x as much but they’re quoting 2.85Ah and apparently treating 1.5 [peak] voltage as a constant). OTOH, Big Wiki says a normal ‘negative’ lightning strike blows ~1GJ.
Alkalines are inefficient at high loads. They can have about 14 kJ of energy (3000 mAh), but you’ve got to take it out slow.
Lithium batteries appear to be broken by design. I have seen only one that could match the lifespan of a single NiCad. My old iPad 1 is still going strong. But on average you’re seriously blessed if you can get two years from a Lithium cell. My latest NiCad power electric razor is over 15yrs old and still going strong. Most Lithium cells seem to have significantly shorter lifespan in just the first month of use. I hate them with a passion. Yet another example of new tech that can’t match the old.
With NiCads you just need to make sure you use them up. Pretty simple.
Well… off to find the third set of LiPos for my 6yr old laptop. These are only good for about 3mins.
Yah, you could do your own common sense battery management on most NiCad an NiMh, but now you’re fighting the smarts or idiocy of the chargers on Lithium types. I can’t figure a really solid solution to keep them alive, taking into account all the variables. Best guess is to not to let them run too low too often. Also not to let them stay 100% charged too long either, but you’re at the mercy of aforesaid “smarts” or lack thereof on most laptops.
Laptops are notorious for this. Folks who leave them plugged in all the time have a warm, fully charged battery (the worst combination for Li-ion) just sitting there dying, and after a couple or three years it’s toast even if it’s never once been discharged. Thinkpads are the only ones I know that can be set to only charge the battery to, say, 75% and not start recharging until it falls below, for instance, 65%. I do exactly that on my X200, and I can fire off a simple command to initiate a one-time full charge if I know I’m going to need a full run, otherwise I have enough for an unscheduled outage or travel and my battery isn’t slowly losing life just sitting.
Exactly! Laptops are best at killing their batteries.
Dell laptops (some of them) allow you to set up custom charging/recharging thresholds. I have E7250 and my profile is charge to 85%, start recharging at 80%. Li-ions are happiest when kept in 40-60% state of charge. Up to 80-90% SOC is still quite healthy (cell voltage up to 4.1V). Keeping 100% SOC is deadliest for cells. Fast charging also degrades the cells.
I don’t see any advantage of ni-cads vs li-ion. I’ve converted several power tools from ni-mh/ni-cad to li-ion and capacity increase as well as output power boos is insane. My old 18V ni-cad pack gave 400mAh out of original 1300mAh at the end of life (it was 3 or 4 years old and took somewhere around 30 charge/discharge cycles). 2 cells in the pack died prematurely rendering whole pack unusable. 5s li-ion on Samsung INR18650-25R has 2x more capacity while being 50% lighter and quite a bit smaller. It also has significantly more “kick” due to way smaller internal resistance. It has so much capacity that I hardly ever have to charge it so it will never deteriorate enough to be replaced.
You have to be seriously misusing/abusing your lithium cells if you only get 2 years out of them. Sounds more like an example of an old man who can’t understand modern technology.
Come on, you don’t have to be old… the people at LG who customized Android for the original Stylo decided that you needed to be reminded to unplug your phone once it was fully charged, every single time it hit 100%, “…to save power”. But it doesn’t. So all that LG accomplished is to misinform and teach 95% of people to burn through their battery’s lifespan more quickly, as if having it always charging-or-discharging should be normal. And I still run into (not old) people who tell each other that leaving your phone on the charger (powered by the charger(!!)) is the thing that wears out the battery. I don’t even really care about phones but I’m frequently unhappy about widespread && wrong ideas about things. Oh, hey, that sounds exactly like I’m *getting old*. Fun.
Heh, LG were still doing that, Optimus Net, Eclypse, L90, G4 all the LGs I’ve had.
There is a slight problem with the statement above – keeping li-ions at constant 100% state of charge IS killing them! That’s the main reason laptop batteries die so fast. Keeping devices with li-ion batteries connected to chargers for extended period of time is wearing out the cells. It is better to disconnect them and let the battery discharge slowly.
https://batteryuniversity.com/learn/article/how_to_prolong_lithium_based_batteries
> Keeping devices with li-ion batteries connected to chargers for extended period of time is wearing out the cells.
Is that why their datasheet specified how many times you can expect to empty and fill them before they are finished? They’re just going to slowly turn into garbage anyway, more quickly if you really do *use* them. I try not to. My secondhand smudgephone is still idling for around a day on a full charge and it was already a couple years old when I got it just over 2 years ago. That may be mostly because I don’t talk on it much, or stream 1080p up to YouTube as much as someone else– but leaving it on a charger for 12+ hours at a time doesn’t seem to be blowing it away, either. It’s getting old as I expect but not very bad, yet. Granted, I never knew what it was like when new so I don’t have much for a basis for comparison.
> stream 1080p
I mean that I don’t stream video with it, ever. I do take the occasional video, such as my overweight cat hunting in the backyard, and there’s still battery for that.
Shortly after I wrote this I put the power mode back from Efficient to Balanced and unplugged it. It switched to extreme powersave at 25% (which I chose) after 48 hours of occasional messaging… now after the absolute garbage reception presumably overworking the radio for some more painful messaging & browsing, it’s at 10%. So I think it’s not doing very badly at all, after everything.
Problem is, some devices the charger handles it properly, some devices it doesn’t. You don’t know which until you’ve got a dead battery.
Datasheet specifies loss of capacity in percentage of initial capacity after X full charge-discharge cycles because it is easy to test thin on automated battery tester. Other properties are harder to check so you don’t see them in the datasheet.
You can use apps like AccuBattery (for Android) to get rough estimate of current battery capacity (after several charge-discharge cycles). It can also tell you how many times the battery gets topped up while staying connected to charger. If the battery is not being topped up to often it won’t degrade so much.
… and then there’s tethering. Plug it in and ‘dhcpcd usb0’ or run some silly app because that’s your entire link to the outside world for many months. If it’s actually bad to have a phone keep its battery at 100%, why do they *all* cheerfully do that using any power supplied as if it’s the only thing you could want? (hint: it’s a rhetorical question but I will accept “because phones are horrible” after which you can get off my lawn)
Yes, I’m not young. Been around enough to know things. I’ll give you one of my issues is not knowing enough about the physical makeup of the battery. Its obvious to Lithium experts that if someone has developed the habits needed to maintain a NiCad cell they are death to Lithium. I’ve garnered enough info now that at least I can get a couple of years from some of them. But as this article points out and other comments here, they are extremely fragile: don’t charge ’em up too much or let them discharge all the way, … Oh and they might turn into a bomb on you. I’ve owned some Dells…. ::cringe:: Anybody with a Samsung smart phone?
I see three issues:
1. Nothing instructing on the proper care and feeding of Lithium cells when you get new device X. Everybody wants to claim simple. Lets not bother anyone with the complications.
2. Improper battery controller design allowing the batteries to be charged too much or run down too far. Since they are so fragile this is key to longevity. Obviously Apple got it right.
3. Fragile construction. Something needs to improve to make them more durable. Apple probably has this right too or at least better.
That said all you have to do to kill a LiPo is ignore it for several months. Making them annoying as hell… since they are in several devices that I only take out once a year. So basically I’m down to buying a new battery for each use.
NiCads simply are more durable.
Oh and a “long” run for a Lithium is two years. Usually they are much shorter lived. Purchased a pair of cordless electric toothbrushes (they only had the two pack) about 6 months ago. I make sure to rotate use every month and only charge them that often. They have never got near running flat. One of them is already showing signs of trouble. :-( I probably charge the too much. >:-[
Nobody should have to expend the same time and energy to maintain a battery as they would the care and feeding of a pet.
I mostly agree with you except the part about ni-cads being more durable. Yes, they can survive more charge-discharge cycles but you have to consider that they have way smaller capacity to begin with and have high self-discharge. So in the end they will need at least 3x more cycles to deliver same amount of energy to load. I hate my Phillips toothbrush with nimh battery that discharges way to often compared to my wife’s toothbrush with liion cell that hardly ever needs recharging (the difference is in the order of 3x) while being smaller in size.
Yeah… to me “capacity” does not denote “durability”. However charge-discharge cycles does. I would also count ability to sustain a charge during unused periods and my NiCad experience seems to beat the Lithiums hands down on this. I’ve not seen the “self discharge” you mention. Take my NiCad powered razor: it gets used maybe 15 seconds a day to trim the edges of my beard and gets at least two days off a week. It goes so long between charges I really don’t know how long that is. Months.
One could also probably factor in temperature ranges and other harsh environment scenarios. But to my point in this discussion “durability”, to me, means the ability to survive longer under normal use. Lithium cells definitely have more capacity and some day they’ll get them right. And maybe the real issue is the source of the cells. Vendors buying cells from sources that cut corners.
ChipMaster are you kidding? Liion beats nicad in self discharge by miles. Liion has the lowest self discharge possible. It is an order of magnitude better than nicad. Nicads are the worst together with led-acid. Nimh are slightly better but only special designs like eneloop brand.
Is your toothbrush more than 10 years old? Nicads were removed from market years ago due to toxicity of cadmium. Today you can only get them for special purposes. You probably have nimh in the toothbrush. It is possible that you have quite oversized cell in it so it lasts long. My Phillips dies every 2 weeks which I consider too often.
What’s that gratuitous personal attack for?
For the next article, the intermediate guide, the guidance on low charge rates by Powerstream may be of interest – https://www.powerstream.com/li.htm
“When the charge rate during the constant current phase is low, the charger process will spend less time during the constant voltage tail. If you charge below about 0.18 C, the cell is virtually full when the 4.2 volts is reached. This can be used as an alternative charge algorithm. Just charge below 0.18C constant current and terminate the charge when the voltage reaches 4.2 volts per cell.”
So systems doing eg energy harvesting of low current sources could effectively avoid the need to manage a CC / CV charging scheme. Which is good because it is hard to achieve when you have intermittent power.
Exploiting the attention this article will get, can I get some recommendations for a cheap battery charge controller module suitable for 2S? Almost everything seems to be based on the TP5100, is there another alternative?
My selfish question notwithstanding, some of the best hardware tips I’ve received have been from reading hackaday comments. So don’t burn me at the stake right away please.
“RESPECT THE LIMITS”
That entire section applies to all batteries, not just Lithium. Lead Acid were known for catching fire during transport and installations with large lead battery banks carefully monitor their hydrogen in the atmosphere. NiCad introduced Thermal Runaway a few decades before Li-Ion. NiMH has some of the safest characteristics just the lower power density.
I was wondering if there are any Li-Ion battery manufacturing being done in North America?