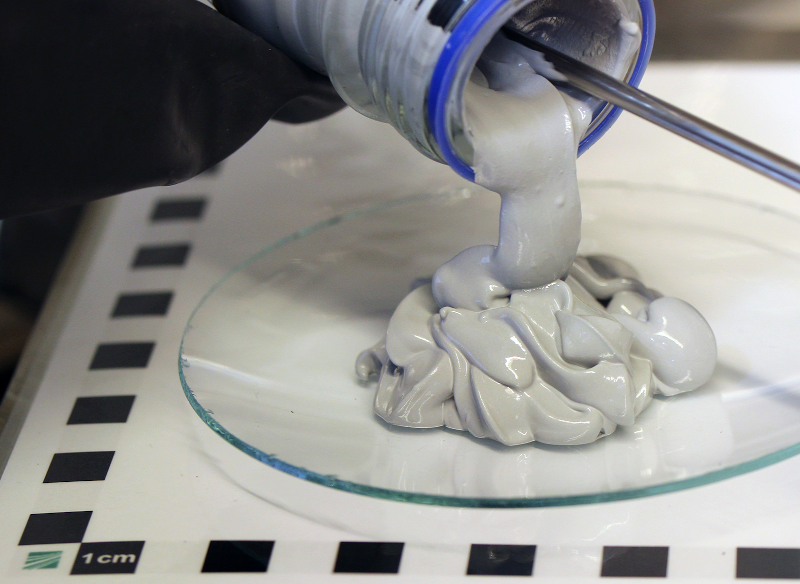
We’ve been promised hydrogen-powered engines for some time now. One downside though is the need for hydrogen vehicles to have heavy high-pressure tanks. While a 700 bar tank and the accompanying fuel cell is acceptable for a city bus or a truck, it becomes problematic with smaller vehicles, especially ones such as scooters or even full-sized motorcycles. The Fraunhofer Institute wants to run smaller vehicles on magnesium hydride in a paste form that they call POWERPASTE.
The idea is that the paste effectively stores hydrogen at normal temperature and pressure, where it stays chemically locked until mixed with water. The researchers note that it will decompose around 250 °C, but while your motorcycle may seem hot when parked in the sun, it isn’t getting quite to 250C.
Interestingly, the paste only provides half the available hydrogen. The rest is from water added start a reaction to release the hydrogen. Fraunhofer claims the energy density available is greater than that of a 700 bar tank in a conventional hydrogen system and ten times more than current battery technology.
One thing that’s attractive is that the paste is easy to store and pump. A gas station, for example, could invest $20-30,000 and dispense the paste from a metal drum to meet low demand and then scale up as needed. A hydrogen pumping setup starts at about $1.2 million. Fraunhofer is building a pilot production plant that will produce about four tons of the material a year.
This isn’t a totally new idea, of course. Using metal hydrides to store hydrogen chemically has other proponents, including French company McPhy energy. There have also been schemes to use other hydrides in powder form as well as chemical hydrogen carriers like formic acid and ammonia.
Will your next drone power itself on paste and water? Probably not for a while although it has been done. If you want to dig into the hydrogen energy economy, [Lewin Day] can fill you in.















What happens to the leftover magnesium? Would it be stored in a secondary tank that you empty when refilling?
I googled a bit. The magnesium hydroxide (Mg(OH)2) will be magnesium oxide (MgO) after the reaction. Since the Mg(OH)2 is dispensed via a pump on demand, the MgO would, presumably, be stored in a waste tank that would be sent back to the manufacturer to be reprocessed into Mg(OH)2. I suppose one solution would be to sell standardized cartridges that contain both tanks and you would “refuel” by swapping cartridges. Another way might be to install both tank permanently in the vehicle and use a mechanism with two hoses at the refilling station that would refill the Mg(OH)2 tank while draining the MgO tank.
If I were designing it, I think I’d go for a cardboard tube like you load a grease gun with, but a bit bigger, similar mechanism to feed it. And the MgO I’d have it crap into a “colostomy bag” for recovery.
This is the way of the future. Have you seen how they load fuel in any Sci-Fi film?
Problems left to solve:
– The paste shoul be glowish yellow in a glass tube.
– The hidrogen should be fused, not burnt!
Fuse hydrogen
There’s only the issue that you cannot let the paste come to contact with air, because it will spontaneously combust with humidity. The tube must be hermetically sealed and under an inert gas. If you get a fire, you have to smother it with sand, because water or CO2 will just make it angry.
The second issue is that the waste product MgO is a solid, so it produces a kind of abrasive paste that is difficult to pump around. It forms small solid particles in a suspension much like silicon paste, and form what I’ve seen in the industry, the equipment to pump it around is more or less perishable goods. You keep replacing the hoses and impellers as they wear out.
The third issue is that magnesium hydride/hydroxide is caustic and corrosive. Handling it requires protective gear, and any skin contact should be avoided. The safety code is 3-3-2 (diesel fuel gets a classification of 1-2-0) so it’s unlikely you can even hand it out to public use.
The fourth issue is that producing and recycling magnesium involves burning the waste material in a big gas fired calcinator and then using a carbon shift reaction to produce magnesium chloride, which is then electrolyzed into pure magnesium and chlorine gas. This process uses fossil fuels, and even if the calcination step was done in an electric oven, the chemical reaction still requires carbon and produces CO2. Plus, it’s ridiculously inefficient as a storage system: probably not more than 10% of the input energy ends up at the output.
Shouldn’t be any worse than lye, excuse me while I go and tell my cardboard can of drano crystals that it should be exploding right now.
You can buy metal Sodium easily – without any restriction – , which have 3-3-2 too.
Yes, you can buy sodium hydroxide, sodium, and other similarly dangerous chemicals without restrictions in small amounts – but they were also common household hazards back when we used to use straight up lye to wash our clothes, with many people getting chemical burns, kids drinking it thinking the white solution was milk, etc. and having their throats scarred shut. There is a reason why we don’t use these chemicals as common household items anymore, and dispensing extremely caustic and combustible chemicals out of a nozzle at a public pumping station is obviously not a good idea. Spilling a bit of gasoline on you makes your clothes stained and smelly – spilling a bit of magnesium hydride paste on you makes your clothes go away.
And if your drano crystals can be stored in a cardboard box, they are not pure anhydrous sodium hydroxide as it would pull enough water from the air to melt into a puddle and leave the box.
“There is a reason why we don’t use these chemicals as common household items anymore”
Might want to avoid such blanket statements. Also, check the label on your eyedrops.
And not for nothing, Chlorox. And Windex; the blue means it’s blueberry flavored, right?
Yes yes, there are dangerous and poisonous chemicals still at home. You can toss a varnish rag in the paper bin and burn your house down.
The point is the incidence of serious accidents has fallen dramatically after most people stopped using lye, sulfuric acid, 30% hydrogen peroxide, raw ammonia etc. for simple household tasks and substituted them with safer alternatives. Especially ones that would emit hydrogen gas and spontaneously combust in air.
If the proposal is to dispense exactly such chemicals out a drum at fuel stations, or squeeze them out of a disposable tube like toothpaste, you can immediately see where it would go wrong. For example, consider the connectors and how to seal them from air.
For processing the used paste, I found a bit about the use of magnesium hydroxide in water treatment plants.
” as a result of solubility, magnesium hydroxide is difficult to handle. Magnesium hydroxide is a slurry that will rapidly separate from solution. The storage tank must be constantly agitated and chemical delivery lines must be kept in motion. Typically recirculation loops are employed with a metering valve, inline, for chemical delivery. Static lines are not acceptable because the slurry will separate, and lines will plug”
Tell me again when people used lye to wash their clothes? You may be thinking of lye soap, but lye soap IS NOT LYE, just as cheese is not rennet. It is a fat that has been saponified by a base, hence the name “lye soap”.
No one’s using hydrochloric acid to clean concrete anymore? TSP to prep painting surfaces? Gosh, I feel so safe.
Even sand can react dangerously with burning metals. For one, it may contain moisture, which means water and kaboom. And magnesium can actually redox with silica (main sand constituent), although not as exothermically as with water or carbon dioxide. Yet still not something I would like to try on my desk. There’s a video of this reaction on Wikipedia: https://en.wikipedia.org/wiki/Magnesium_silicide
>No one’s using hydrochloric acid to clean concrete anymore?
Muriatic acid is sold diluted to 37%) with the assumption that you would dilute it yourself. You can still buy it, but for safety reasons they no longer carry the strong stuff for ordinary consumers.
Oops.. formatting bug strikes. Muriatic acid for concrete cleaning is sold diluted to less than 10-15% these days, while back in the day you could buy it stronger than 37%
>when people used lye to wash their clothes?
I would say the use of lye for washing clothes gradually faded away after the rationing ended in the mid 1950’s. Soap and detergents were expensive, so poor people kept using straight up lye for a long time, including for making their own soap.
Magnesium Hydroxide, or Magnesia as it is know, is an over the counter indigestion relief product called Milk of Magnesia in the UK.
Muriatic acid typically is HCl dissolved in H2O to approximately 31.45% concentration. Hydrochloric acid is at full strength right around 37-38% as the water cannot hold anymore HCl gas in solution.
My grandmother used lye often, but rarely if not ever to clean with. It was used to make soap from cooking grease as many poor rural families did back then. Also used to break down waste in lye pits, due to the lack of trash disposal services. They’d burn whatever else remained.
I’d have two bellows bottom-to-bottom in a canister, one empty, one full of charged paste. The device that harvests H2 from the canister pumps back the processed sludge in the empty bellows, taking up space freed by removing the charged gloop. Canister are sealed, so no oxygen comes into contact with air. In the charging station, canisters are swapped and you are charged based on the ratio of the position of the bellows bottoms (ie. you can swap even if the canisters aren’t exhausted yet). And it should be easy to carry more than one canister, either for battling range anxiety or for extending range.
As the paste creates large amounts of Hydrogen when exposed to water, I feel that a more substantial container than cardboard is needed to avoid accidental high concentrations of Hydrogen gas. A nightmare would be an Amazon lorry load of cardboard tubes of the paste overturning in the rain.
Reusable steel cans are probably the answer.
It is reassuring that the waste product, MgO is a relatively harmless substance, although it should be recoverable for conversion back into paste or even to make Milk of Magnesia for indigestion!
What converts the magnesium hydroxide to magnesium oxide? Is the water removed because of the high temperature that the device operates at?
No, the water gets removed elsewhere in a big gas fired oven when the magnesium gets recycled back.
There would be lots of interesting stuff to say about this material, but the messages keep getting flagged down and censored automatically, because they contain certain bad words that you’re not allowed to say.
Its HYDRIDE, not hydroxide.
MgO? After which reaction?
Are you sure that’s right? Mg(OH)2 is a very stable basic compound and is the main component of Milk of Magnesia that’s both an antacid and laxative although real “magnesia” is the oxide.
It seems much more likely that you’re referring to the hydride
https://en.wikipedia.org/wiki/Magnesium_hydride
which decomposes to release hydrogen upon adding water just like the article states this paste system works.
Exchanging tanks just isn’t practicle, imagine my 81yr old mother swapping fuel paste tanks??
Well said…..either way, swapping feels lesser cost effective but both seems logical……so little time…… what about the deadline 2030, like a stretched 2030 for ICE vehicles, manufacturers actually still braving into their engine bays huge donkey Kong V8’s(are manufacturers going to rebate every single ice vehicle owner for their vehicles in 2029?- really wondering) so I really wonder when the time actually arrives, one would think that hybrids would be the way until EVs and better yet Hydrogen Vehicles hit the shores.
PS. only the first line is relevant to your comment, just needed to get this view through to all of us
I found some more information at:
https://www.zess.fraunhofer.de/content/dam/ikts/zess/documents/POWERPASTE_WHITE_PAPER_2019.pdf
In the flow diagrams there doesn’t seem to be any arrow showing where the waste magnesium hydroxide goes. I couldn’t find anything about this in the text. Presumably this waste magnesium hydroxide would also contain residual magnesium hydride which might still be flammable?
Oh, you’re right, it’s MgH2 -> Mg(OH)2, not Mg(OH)2 -> MgO.
Well, the last step happens when the hydroxide is dried out in a gas fired kiln at the recycling plant.
I’m more concerned about how you separate out the waste MgO from the active paste/water mix in a continuous process.
Gwyneth Paltrow’s Goop !
Better than her candle!
She knows where to stick it.
How efficient is the process overall? You have to make the magnesium hydride somehow, and if it takes a lot more energy than your recover in the vehicle it’s not going to be an appealing option.
It’s appealing enough even if the whole cycle isn’t that efficient, it will have a niche as it looks rather convenient and easy to distribute.
No reason why this should be particularly bad, though its not going to set the world on fire with efficiency either, as even if it matched a battery (which would be stupendously impressive and surprising) in the energy in vs energy out, this goop will have transport costs far greater than running some electricity down the grid..
But something ‘green’ that produces no harmful gases in use and can be put in place of the petrol powered strimmer/chainsaw/lawnmower/bike which the serious users need much more endurance and shorter down time than a battery can provide. It might not be the best option for everything, but it looks pretty viable to me – the only questions are how heavy and how small such a system will be as to it might not win over other techs once the engineers have had the chance to study and build around the one they like best. (For instance if its too heavy or bulky swappable batteries and the charger on the truck might work best for most tools)
> its not going to set the world on fire
It might set you on fire though. It’s quite a hazardous chemical, with a tendency to self-ignite in air and chemically burn you on contact.
And all the various acids, alkali, fuels and industrial gasses we ship around and use are wonderful for you – nothing humans do at all is perfectly safe from failure, but that doesn’t mean its going to be a catastrophe when used if it’s used right.
Its that last part that is all that matters, is the engineering and maintenance done correctly to make it safe enough. In the case of risker tools/processes etc we tend to have the right safety equipment with them to make it safer anyway, which we don’t bother with on everything else…
Where does the MG2 come from?
Yes, even dioxygen difluoride can be safe when it’s used right.
But when not, better have your running shoes on.
https://www.greencarcongress.com/2006/01/safe_hydrogen_r.html
“The slurry, both before and after yielding the hydrogen, is not flammable, safe to handle, easy to store and can use current pumps and tanks used for diesel fuel, gasoline or water. The slurry is reacted with water to produce the hydrogen required. The metal hydroxide byproduct is captured and recycled.
The slurry consists of a finely ground light-metal hydride, protected by mineral oil and suspended by dispersants to keep the particles from settling out of the suspension. (Safe Hydrogen originally developed the concept using lithium hydride, but is further developing it with magnesium.)
The oil forms a protective coating around the hydride particles that slows the movement of water toward the particle.
This protective coating allows the hydride to be safely handled and stored in the air without absorbing moisture from the air. It also slows the kinetics of the reaction allowing the development of reaction vessels to mix the hydride with water for releasing hydrogen.”
What’s the degree of reusability for this setup?
Aka is the magnesium compound that results from this reaction easily collected, stored, unloaded when you fuel back up, and then re-processed back into fuel? If this full cycle chain isn’t possible at a high effeciency rate, then this system is a rough sell. Magnesium isn’t exactaly as easy to get large quantities of (compared to petroleum for example) so single use is obviously out of the question.
“the paste is easy to store and pump”.
I doubt it. At least not as easy to store and pump as a homogeneous liquid like petrol.
I think this is a gimmick.
Easy to store and pump compared to *gaseous hydrogen*.
Yes, quite possibly. Although in some ways gases are quite easy because you just need to open a valve from a higher pressure container (which obviously has its own safety issues especially if it’s highly flammable like hydrogen). I think that liquids are probably more convenient for private vehicles, and hydrogen gas might be more convenient for commercial/industrial applications. Maybe this paste fills some intermediary need.
Petrol is a no go! Not only is petrol only available in naturally finite (and non-renewable) amounts, but its exctraction, transport, refinery and consumption/combustion is an ecological disaster. One doesn’t need to be a die-hard tree-hugger to realize that!
Hey science Newbie, do you remember of what is made petrol ? That’s right, it come from crude oil.
It’s made of fossilized vegetals and animals that was faster and faster decomposed by the heat and pressure as far as it goes deeper and deeper…
So, since mankind did’nt explore and urbanize all the planet, this natural process is still running for the obvious reason that nobody can’t stop decomposition.
And, technically as historically, that’s the first renewable energy that does’nt require human intervention at any moment.
Of course, it still pollutant, but don’t get me wrong, i said “renewable”, not “environnemental-friendly”
Fun fact: the Venezuela is the first geological reserve of crude oil (with a volume counted in billions of oil barrel) and, despite that, because of deeply badly dumbs at the government, the venezuelians must import their petrol.
Petrol resp. crude oil may be “renewable” to some extent, but it takes millions of years to generate. Way to slow to keep in pace with the speed at which mankind is depleting natural reserves…
… Except you forgot a detail: it (will) never get stopped by anything or anyone, so it’s still supply crude oil even if we look for a better/greener/safer way to get combustibles for our engines (so we even may encounter large issues of ground sinkings and crude oil floods because of this still processing decomposition and if we can’t handle all the availables ressources of this now, it may even become worldwide issue since it’s happen everywhere on earth)
There may not be more oil in the future at all – it takes the right environmental conditions to have enough life die in the correct situations and geological zones to get buried and eventually cook up more oil. Its fairly certain somewhere, somewhen in Earth’s future this will be true again, but its not like any old dead thing will create oil eventually, the world is a very different place now to how it was when our current crop of oils was laid down…
I’d like to see an article explaining how the dinosaurs got from the surface to points a mile or two down.
“Oil is made from dinosaurs” seems like something silly you might tell a child. It does make a good story.
Fossil Fuels are formed in a geologic time scale, which is something far slower than human civilization can function at. The time necessary to form a deposit of petroleum exceeds all of human history many times over, so for all human intents and purposes, it is not “renewable”.
Even then, it only forms under specific conditions, which is why there aren’t oil deposits basically everywhere, especially big ones.
What kind of “human intervention” are you talking about, because drilling a well to get petroleum and natural gas or digging a coal mine definitely require the actions of humans, and that’s not even taking into account the requirement to refine it if it is to be used as fuel.
Except coal, it’s pretty much the same as when it was dug out of the ground other than being made into easier to handle sizes. (And full of contaminants, which is why the coal deposits of more pure quality get the premium prices, and even then they’re still dirtier than the non-coal options. There’s no such thing as “clean coal” outside of marketing pitches.)
Venezuela has issues, lots of issues, but that’s probably best discussed over on a political forum. ;)
Anyway, thanks for reading to anyone that got this far.
Petrol is easily made from gasses found in the atmosphere. Just add a solar or wind farm to provide the energy.
Exactly what I believe is alot easier than trying to handle hydrogen. Create simple hydrocarbon gas like Methane from the atmosphere and drive on it like CNG cars already do. The energy required should be available in abundance. We need practical solutions now to get something going in the next decades, not yet another “revolutionary” idea that leads to nothing.
There’s a breaking energy story that could potentially be a repeat of Texas’ multi-billion dollar Midland Basin…
A basin that has been producing since the 1930s and won’t reach peak production until 2035 at a phenomenal 3.8 million boe/d …
The most trusted name in natural resource assessments—Wood Mackenzie–says this new discovery is analogous not just to the giant Midland, with a development value of $540 billion, but to two other world-class basins.
In fact, it could become one of the most interesting stories of 2021.
One of the key men behind this play is former Navy Submarine Force Yeoman Dan Jarvie, once searched the depths of the Pacific for submarines…
But today he is one of the most respected petroleum geochemists in the world…
And what he is uncovering could change the entire African energy sector.
A 6,000-foot-thick Permian basin that could prove up 120 billion barrels of high-quality oil and gas in place.
A MAJOR NEW PERMIAN DISCOVERY IN 2021?
During the Permian era, there was only one continent…
And the earth’s vast ocean was teeming with marine plants and animals.
This organic matter fell to the bottom of the sea… and over time turned into high-quality thick oil.
The world’s most famous Permian formations are currently in the US.
If this turns out to be true, I hope they don’t (waste) flare off the Natural Gas, just to get the oil.
Saudi Arabia did that for years (of course, I’m sure their foreign investors promoted that idea)
During the early 1980s (and I have no idea how many decades it occurred) the Saudi’s flared enough Natural Gas _EACH_DAY_ to meet the Natural Gas needs of the British Isles for 30 years.
IF, AGW is true, that was probably a major source of atmospheric CO2.
I remember flying over Saudi Arabia one night in 1987. I thought I was seeing the Sun rise, only to find out (after watching the horizon for maybe 15 minutes) to find it was just Natural Gas flares.
Yah, flaring the gas ought to be criminal. I don’t care if you have to get rid of it by camels towing zeppelins to a terminal the other side of the desert, build a gas fired aluminum furnace on site, or a giant gas turbine for electricity, but no flaring.
They (Saudis) have little concern outside of their tent.
My father in law worked the fields back in the 1950s.
Oil change on a generator was simple. Open the drain plug, add oil in the top.
All done without shutting down. No concept of waste or environmental damage.
[RW ver 0.0.1]
I could be wrong, but I think they use it to make fertilizer and other products now.
Fertilizer, ah, yes, the sh*tty product that still sells.
Los Angeles uses the sewer solids to create methane, powers gen sets, and sells the power to the local utility. (Edison) The remaining solids, (after de-watering) is sold to Kellogg and bagged up for the public, sold at Home Depot, and Lowes. It’s a vicious cycle.. (20 years there)
[Jerry]
Milorgamite.
(Milwaukee organic fertilizer)
is made the same way.
That’s what people said about fire, agriculture, riding horses, the steam engine, the automobile, radio, personal computers, mobile phones, smart phones.
There is mature technology out there for pumping materials like concrete, which has to be a much harder problem due to the size of the aggregate particles that go along with the cement.
Concrete pumps have lifetimes in the very low hundreds of hours and many use a pressurized inert gas fed from a cylinder to protect the seals/gear train.
Reminds me of the old Soviet salt train somewhere out in the salt plains of Mongolia. Saw a documentary about it; they laid a big looping track over the salt and put a locomotive on it, with a drill that dips into the salt and pumps it up to a train on parallel tracks. When they’ve done one loop, around, they yank the tracks six feet to the side with a bulldozer and start again. It takes them 25 years to move across the lake, by which time enough salt has precipitated in the old dig that they can start all over again. The locomotive and the train have been going their rounds for more than 50 years now. There’s two guys there whose only job is to slather grease on everything, all day long, and when they’re not doing that they sit in the cabin and play chess.
As with all things that promise to revolutionize ${industry}, I’ll believe it once it actually has some amount of market share.
And this is superior over binding hydrogen to carbon how? Simple hydrogen-carbon fuels can be easily synthesized too (e.g. methanol, ethanol). They’re clean burning, can be transported and pumped using well-proven technology, and can be produced without pumping dead dinosaurs out of the ground.
You’re forgetting the top benefit of having “hydrogen” in all these tech demos for the last 50 years: it grabs the imagination of the non engineers in charge of funding grants.
Yep, the politicians and funding agencies love a gimmick. Maybe the petrochemicals people just need to rebranded hydrocarbons as as carbon hydrides?
I’m sure they’d love ethanol better.
Well it’s easier to get the farmers behind. “Sell us your grain at market prices” to make ethanol gets a lot more support/votes than the “give us all your organic waste, straw, chaff, dung, pulled weeds, grass clipping, hedge trimmings…” and we can make methanol from it.
The whole point of this is to not emit gaseous carbon into the atmosphere.
Yeah, but what is the source of the input energy???
Overproduction caused by solar or other green energy. Just another “Power2Gas” technique
Except the production and recycling of magnesium involves a gas furnace calcinator and a carbon shift reaction that both produce CO2
What someone really needs to invent is a simple binding molecule with no solid waste product…say Oxygen. Wow I just solved the world’s energy problem…just bind two Hydrogens with an Oxygen! Stable at STP!
Too dangerous. It kills thousands of people every year!
Yes, DiHyrdoMonoxide. DHMO, a common industrial by-product, has been found in cancerous tumors as well as in spontaneously miscarried fetuses and full immersion is a leading cause of death.
That’s just using defamiliarization to make hydroxylic acid sound more dangerous than it is, I mean it corrodes a lot of stuff and dissolves a lot else, and it has been used in torture, but as long as it is carefully handled and you don’t ingest more than 10 standard units daily, it probably won’t kill you… unless you happen to be holding metal when it does one of those megawatt discharges from particles in atmospheric suspension.
+1000
Why try to create an entirely new distribution infrastructure when the existing one can be reused.
And both the carbon to bind the hydrogen to and the hydrogen itself can be obtained in gaseous form in the atmosphere. Just add a solar or wind farm and presto.
BTW, petrol batteries (tank) last decades longer than electron batteries.
Gaseous hydrogen from atmosphere? Not in any meaningful amount, and even that at great cost and energy.
How do you treat a fire of such a thing ?
A common question. Any large storage containment will have appropriate measures in place. Same for any large tank of CNG. In usage, the amount will be limited and IF ignited, will burn out quickly. There are several fuel cell autos running around now. So far, no worries.
That’s dodging the question.
Real answer: you smother it with sand because water and CO2 based extinguishers both sustain a magnesium fire.
The compound is also highly caustic/corrosive and abrasive to pump because it contains suspended particles of solid MgO, and it spontaneously ignites in air from any moisture.
There’s no elemental magnesium involved in this: it’s Mg++ throughout. All that changes is whether it’s swapping electrons with hydrogen or with hydroxides. The magnesium has already done its exothermic thing so it’s no longer the problem.
You do have a problem that heat makes this release hydrogen, which in a fire is a positive feedback loop, but gasoline increases its volatility with heat as well, so that’s not much different.
This is a hydrogen fire rather than a petroleum fire, but it isn’t a magnesium fire.
Same difference. Check the safety datasheet for magnesium hydride: sand or dry extinguisher only, no water, no CO2.
And, it is a magnesium fire once it gets going. The material decomposes at 287 C producing plain magnesium and hydrogen gas.
Australian company, LAVO, working with the University of NSW have released hydrogen generator and storage for home using a hydride.
Last time I bothered to annoy a Prof with this question the answer was: “not that practical”.
Even pure liquid H2 has around 50% the energy of petroleum (larger fuel tanks), is usually derived from natural gas, and costs 14 times the price of regular fuel per km. There is only 1 advantage of pure H2, and that is the mass at lift off.
As everyone is going electric soon, the reality of transmission efficiency losses and generation options remains unsolved. If you still think solar/wind can support the demand, than one really hasn’t looked at the reality of the limits charging 300 million batteries will create.
I like the generically engineered algae+crustacean option that closes the short-term carbon cycles using areas that do not impact normal agriculture (-3’C to 10’C, and >37’C arid zones), treats run-off to mitigate eutrophication of waterways, and scales up well. B100 diesel from microbial sources in theory (30% by mass achieved in 2014) also cuts Sulfur emissions to negligible levels, and has energy densities comparable to classic diesel fuel.
This technology seems to offer some advantages:
1. can be appended to existing wastewater treatment facilities
2. is a traditional petroleum product that can be stored, handled, and consumed by existing infrastructure
3. is carbon neutral and should not negatively impact global warming models
(there may be some controversy here depending how the reactors are implemented)
4. countless ways to implement a plant means patent encumbrances are nonexistent, and small Spirulina farm examples are already commonplace
You will eventually learn that most problems society faces are not technological, but rather social or political in nature. Eventually people may reach the same conclusion, or discover something better along the way by rare consensus. Personally, I wager the future is likely wifi-enabled goat-drawn carriages for all. ;-)
With the right energy storage and smoothing methods, and a push for increased power efficiency getting all our energy from the sun relatively directly isn’t actually that far-fetched. I don’t think it will happen any time soon, but its certainly possible (and desirable we should get there – almost all the energy in the planet comes from the sun various degrees of directly, and we can’t forever burn the leftover chemical potential from prehistoric times, eventually that will run out (and it might well cook us all to death doing so too)).
I don’t think everyone will really go electric for quite some time, so the bio derived fuels are bound to play a big part in the near future.
I think you are also missing another major advantage of hydrogen, it doesn’t take significant time to refill the mobile units, or need precious and rarer elements to make the battery tech.. Even if it only gives you 1/3rd the range of a petrol powered similar vehicle the plug in and be ready to go almost immediately makes that a pretty small issue for the user, and vastly better than battery.
>With the right energy storage and smoothing methods, and a push for increased power efficiency getting all our energy from the sun relatively directly isn’t actually that far-fetched
Yeah, but that’s skipping the part where you come up with the right energy storage and smoothing methods, economically and sustainably. Not like today when we’re sourcing Cobalt from Congo and Lithium from China, in a once-through process that results in a huge pile of discarded batteries that are never recycled because of high process costs relative to virgin materials.
That’s been my point to my neighbor who thinks electric cars are the future. My wife and I point out that the power that is supplying his car is sourced from Coal or Natural gas likely. Even though he’s leased solar panels on his roof (purchase, sure…lease??? WHY??), it’s not enough energy to power his 2 cars. My Prius on the other hand is efficient and low polluting. I also don’t have to charge up at special places.
I had read once that reformation technology existed to take carbon from the air, and using electricity and heat from a nuclear reactor it could produce Octane for fuel with no carbon generation. The fuel itself would be carbon neutral due to carbon capture used to form it. This method of generating petrol would only cost about $4 USD / Gallon and be carbon neutral (and likely be used to clean up whole sorts of nasty atmospheric contaminants).
So, portable power needs to be relatively safe (no energy source is 100% safe), energetic (so forget ethanol) and green (which is if’s reformed from carbon capture and water it is exactly that). Why are we insisting on using batteries that will need to be handled as a waste product in 20 years instead of working on hybrids that can use $4/gal naturally produced gasoline from carbon capture? Electric vehicles aren’t necessarily the answer. And I don’t like the idea of dealing with lithium waste when all this comes due in the decades to follow.
Lithium is more likely from South America…huge mines there…
Actually, Australia has taken the lead
If/When battery demand outstrips supply (which it should if EV’s and all our other personal electronics continue to demand) the recycling will come to be cost effective, even cheaper than virgin materials – its already highly refined source material in cans/packets…
So while our lack of recycling is stupid and it would be great if it was being done already it should just happen when the economics catch up – same thing with cheap refurbed EV’s, if the market keeps growing as it is new/recycled batteries in old EV’s will start to become cheap for the consumer, while still profitable for the garages doing the work.
>when battery demand outstrips supply then recycling will come to be cost effective,
In other words, if batteries become more expensive than they are today, recycling them would make sense. However, batteries must become cheaper for all the EVs and gadgets, and grid energy storage to take off.
The irony is that we can only afford these technologies while they are manufactured cheaply using fossil fuels and single-use chemicals out of easily accessible mines. Closing the loop and turning the outputs back to inputs is either economically infeasible, or the technology to do so doesn’t exist. E.g. how do you use wind energy to make concrete to build a factory that makes wind turbines?
The major advantage of hydrogen fuels is that you can burn them and get heat without involving carbon.
Almost everything else about hydrogen is a huge stack of problems compared to carbon-based (or, by far the major case, carbon- and hydrogen-based) fuels.
Just because you avoid carbon doesn’t make it clean. Does your system captures the Oxygen when splitting off the Hydrogen, and use it to burn the Hydrogen? I have never heard of any of the Hydrogen advocates building a system that does this. So, back to Chem 101 – burn Hydrogen in air, and you do NOT get one output of H2O, you get 2 outputs: you also get NOx pollutants. The pro-Hydrogen propaganda machine is totally into only telling one-half of the truth. And half-truths never round up.
Probably not going to be a very popular idea but, given that we are probably not going to cut down CO2 emissions very quickly, pumping sulfur/sulfates into the atmosphere might end up being a practical way to keep temperatures low?:
https://en.wikipedia.org/wiki/Stratospheric_aerosol_injection
I’ve seen reports suggesting that the covid-related reduction in industrial activity has counterintuitively led to increases in atmospheric temperature, due to a reduction in sulfur emissions.
if you don’t mind losing all forests in the process it’s perfectly doable…
“it doesn’t take significant time to refill the mobile units”
Except for the transportation/removal/storage of a spent abrasive ceramic slurry, and the efficiency lost with a centralized reclamation process. Or between 2 to 6 times slower than existing fuel transfer operations at best. ;-)
I don’t understand how adding more steps to an impractical H2 based system makes it better. I understand the paste is intended to make it easier/safer to handle the H2 as a material complex, but it doesn’t really solve the entire use life-cycle or degraded energy density issues of the H2 itself.
Anecdotally, after the local liquid H2 powered bus trials ended, most jurisdictions scrapped the fuel cells and quietly reverted the platforms back to diesel. Asking groups to change their behavior is hard, and adding a sustainable drop-in substitute is likely the only practical solution…. especially if that option is more economical and sustainable.
YMMV, but I am sticking with the goat-cart prediction for now… ;-)
“What’s he doing?, That’s pure capacitance gel!!!”
Before you all get excited, what’s the round trip efficiency from renewable energy to producing magnesium hydride to fuel cells back to electricity? I am estimating less than 30% round trip efficiency. Compare to rechargeable batteries that have 90% round trip efficiency. In this era where efficiency has high priority, wasting 70% of generated electricity is unacceptable. The Physical Chemistry of this one cannot be significantly improved in terms of roud trip efficiency.
I presume they could use the same regeneration cycle as…
http://inventorspot.com/articles/japan_magnesium_energy_cycle_5887
A barrel of paste warmed up to 245 degrees and set off with a small primary….
A barrel of gasoline not even warmed up and set off with a small primary…
The barrel of MgH2 would detonate and disperse instantly, leaving behind a very large flammable cloud of hydrogen and magnesium vapors.
The barrel of gasoline would make a nice movie prop explosion, but it wouldn’t level the entire block.
Gasoline won’t dissociate like a hydride will, it will just vaporize and ignite.
https://www.youtube.com/watch?v=-QUMX2Y4I20
The gasoline vapors require oxygen to burn, so the rate of burn is limited to how well you have dispersed the fuel. In order to make an effective fuel-air explosion out of a barrel of gasoline, you already need to have a fair amount of high explosives in the barrel to spread it around. Otherwise you get a big flame and a mushroom cloud – a flash but not much of a bang.
A chemical like hot magnesium hydride can rapidly decompose into a gas and disperse itself. Magnesium hydride is in fact used in some high explosive mixtures.
All true. Liquids do not burn, they vaporize and then they burn, and if contained, may explode.
The best way to extinguish a lit road flare is dunk it into a bucket of diesel fuel.
https://www.youtube.com/watch?v=A8C6G29nQsc
No distrust lingering over MP3?
Don’t get me wrong… this is interesting science. But as is often the case with alternative energy, I’m left with the impression that we’re sitting in a living room, vigorously debating the finer points of pinochle, while completely ignoring the fact that the house itself is in flames.
Hydrogen is NOT an energy source. At best, it’s an energy conveyor or transporter. It has to come from somewhere. Unlike hydrocarbon fuels that can be mined at a net energy gain, H2–in whatever form you wish to store it–has to be manufactured, and that takes energy.
So how do you do that? Electrolysis? Ok. Where do you get the electricity? Hydro? Tree huggers have worked actively to DEcommission dams.
Solar? Solar panels are dirty and resource-intensive (including water) to manufacture, and I’ve yet to see conclusive data that a given panel can produce more electricity in its lifetime than it took to make it.
Wind? Unreliable, kills birds, generates noise, and is a producer of waste in the form of expired epoxy/fiberglass blades that have to be land-filled.
Until we abandon our irrational fear of nuclear power and build a thorium reactor infrastructure, or Bussard’s polywell fusor comes on line, plans for an all-electric automobile fleet are at best self-delusional; at worst, self-destructive.
As Joel pointed out, our automobiles don’t need to be carbon free, just carbon neutral. Biofuels potentially answer that requirement and can be distributed using existing infrastructure.
Re tree huggers: there is an economic *benefit* to removing hydro-electric dams: https://therevelator.org/edwards-dam-removal/
https://www.nature.com/articles/ncomms13728 is an energy analysis of solar panels published in Nature, that among other things says the current energy payback of a solar panel is 1 year, so with an expected lifetime of 20 years, they produce 20x as much energy as used to make them.
If you need more conclusive data than this, I suspect that there is not likely to be any dataset you’ll accept as conclusive.
That’s an interesting article for a number of reasons. If their numbers are correct, yes it seems that the net energy output of a solar panel made now exceeds the energy needed to manufacture it.
Although the cost to the consumer isn’t the manufacture cost, but some indeterminate mark-up of 100-300%. A case in point being that I can buy solar panels in the UK, secondhand, for around £0.25 per watt. But in the US the prices are much higher, maybe 4 times that. (I was even considering starting to export them to the US). We still have a way to go to cut out the Porsche-driving middle man.
Another idea I had is that, once the break-even point is reached, you could start a self-sustaining manufacturing plant in a desert region, that made solar panels for itself and sold the excess. This could be totally carbon neutral, and would eventually expand to cover the whole desert. This effectively exports the desert sun to other areas of the globe.
As for wind power being unreliable, yes so it is, but just make it charge car batteries when it runs and allow people to drive after a windy day. Or use it to generate hydrogen, which you capture (plenty of old gas fields available for that).
>and allow people to drive after a windy day
That’s begging the question that we would be living in a communist dystopia where someone has the power and the right to say when people are allowed to drive.
There are only two “energy sources”: fusion and fission. All our energy comes indirectly from those, in some form or another via a wide variety of energy carriers.
Iceland is an interesting case, where geothermal energy is available close to the surface. There’s probably other like areas of the Earth, but Iceland could provide all of Europe’s electricity if there truly were superconductor cables.
Since there’s not (cables), the use of geothermal to create other fuels in a factory removes the stupid wind farms and solar panels from the equation. You just need to drill for it, and re-use petroleum pipelines for the hot water. Iceland is close enough to US and Europe that supertankers of goop can flow both ways.
The question is, where in the US and China can you drill to magma in the shortest distance. This would reduce the ocean traffic.
I was impressed with Iceland’s building heat solution, with their underground pipes feeding to buildings and heating radiators with boiling water. Then providing electricity to the towns using steam generators putting out megawatts.
There are already Geothermal Refineries currently being used in New Mexico, I don’t know about the surrounding states, however I met a Representative of that Company in Santa Fe some 4 yrs ago, she was working with the State Legislators regarding their operations. So I know we are already involved in Geothermal operations, however they are currently being used in that process to refine petroleum.
The problem with New Mexico, is they are gambling their water aquifer against corrupt politicians. Already the groundwater has shown signs of pollution, but the laws are written that geothermal wells are regulated no different septic tanks. That is, they are allowed to pollute ground water.
There should be no geothermal hot water injection near ground water aquifers. Rule #1.
Wow all the false claims about all safe reliable cost effective job producing in every region energy. Still trying to prove after 70 years that nuclear energy is more of a problem than a solution. US done with nuclear as are most nations who have had it. America Europe Asia moving ahead constantly with a variety of renewable energy storage and advancements in electric and hydrogen power. No investors or banks will get near nuclear investments or r&d. Only Russia is still trying to sell reactors with Indian Iran Saudis who want nuclear technology when plentiful conventional and renewable potential exist for weapons conversion might exist. 1000 watts per square meter average 5 hours per day solar everywhere many regions twice as much production. Wind wher it is prevalent high energy density never depleted requiring equipment moving and site clean up. Hydrogen major element in all advanced nation energy solutions. Generation everywhere from excess grid power use to level load, power engines fuel cell combined heat and power, heavy equipment beyond battery power, shipping revolution from dirtiest bunker fuel to cleanest. Can even be produced by floating power plants with solar wind even ocean energy for offshore in shipping lane Ship fueling.
Windmill blades can be burned and the waste can be used as aggregate. Thing is someone has to chop them up first.
Photovoltaic (energy) rentability *really* depends on where and how you deploy it.
Both wind and solar need energy storage and vast networks to be viable without any other sources. It’s not impossible, just really, really expensive.
A nuke plant can easily win the economic battle, provided it’s given the 20 or so years it needs from start of construction.
Hope to live long enough to see fusion making electricity instead of craters…
you don’t need to use electolosys to make the MgH2 fuel it can be made simply by the following simple reactions
MgO+2HCl=MgCl2+H2O
MgCl2+2Na=Mg+2NaCl
2Mg+2HCl=MgH2+MgCl2
As you can see the byproducts of each step are used in another step.
Wow reading this thread, and everything went in ever possible and impractical direction. From what I’ve read, the paste would be in a cartridge, that you would simply remove and replace at a refueling station. The Hydrogen would be released due to a reaction with water by way of a plunger, that hydrogen would then be used in a fuel cell “battery” to produce electricity, in which the electricity would be used to power the electric vehicle etc. They are saying that preliminary indications show that this process would allow vehicles to go further the petroleum based fuels! The paste is a stable medium that its makers say is 10 times more energy-dense than lithium-ion batteries!
One has to keep in mind the intended use is things like scooters and ebikes.
Well hydrogen paste may be a good idea but the fact is that the best “hydrogen” energy storage chemicals we have are hydrocarbons, so lets forget about working on the hydrogen and start looking at collecting the carbon.
So this will be in some kind of easy to replace cartridge ? Hope HP Printer division doesn’t make it…😄😄😄
Well you’ll be able to buy a car for $39.99 with 20 miles worth of sample fuel.
Then with a fine drill/ prybar/ screwdriver and funnel you can refill that sample container cheaply too.. This sounds like a winner.. Get the car for the OEM price of the fuel refill…
I applaud the tenacity and creativity of these guys to explore potential new fuel options. Surprisingly, so many people even on this forum seem to have very narrow horizons. Come on people! Just because you can’t see how this could work practically or economically right now, doesn’t mean it couldn’t be feasible for some future application. Why be so quick to only criticize? I think this is a really intriguing concept. I guess if the magnesium could be involved in some of the energy storage of the material that would make it a lot more efficient. No point in having a big metal atom in there otherwise unless it can be super cheaply converted back to hydride.
yes but if you drip a drop of gasoline at the pump it doesn’t give you a chemical burn and then try it’s hardest to set the whole fueling station on fire…
Imagine if you ALSO had to refill your battery with sulfuric acid every time you filled your car with gas. And you did it from the same pump nozzle. There would be a lot of chem burns and corroded fill ports.
A chemical burn… From a gas the floats away? How does that happen?
A Number of years ago, Rocky Mountain Institute did some testing to compare the explosive risks of gasoline, CNG and hydrogen in vehicles.
The vapors of gasoline and CNG are heavier than air so tend to sink and pool… And explode rather spectacularly, leaving little of the vehicle usable.
A hydrogen leak, being lighter than air tends to rise and disperse; If ignited and not contained, goes up with a bit of a pop leaving the vehicle largely intact.
Yes, I know about the tank trucks that have exploded… With one heck of a lot more hydrogen that a single vehicle would contain. Ever see a refinery fire?
@Nathan (Literally no idea why this refuses to attach replies to the post I’m replying to. But whatever.)
We are pessimistic because we are critical thinkers.
Problem: Fossil fuels bad.
Solution?: Replace fossil fuels with a less efficient fuel that is expensive(money/power/effort) to make, dangerous, and requires fossil fuel…to….make…..damn it wasn’t looking to bad at first…
Hi Ian, you say you’re pessimistic because you’re a critical thinker, but what if you’re more of a critical thinker because you’re pessimistic?
Of course critical thinking is vital, but all I’m saying is that often a little bit of _constructive_ thinking can go a long way.
What’s amusing, Hydrogen as a gas is quite unstable, and hates being released into the atmosphere.
It immediately combines with O2 and creates a dense cloud of water vapor.
The paste, not so much..
MgH2 readily reacts with water to form hydrogen gas, so if there is a fire you can’t use water on it without the risk of making things even worse.
I love these articles. The majority of vehicles that run “high” pressure tanks, run at 300 BAR (5000PSI). I know the municipal fuel cell buses where I live do.
Sometimes we also see that 10000PSI (700BAR) is cooled, liquid hydrogen, like liquid oxygen (it’s not).
Neither 300BAR or 700BAR is “specially” cooled beyond what is normal for coolling compressed gas.
Put your hand of the air compressor at a gas station sometime. It does warm up some…. Not enough to need
refrigeration.
For the purposes of illustrating scale for these pressures:
SCUBA dive tanks come in two version; SCUBA low and high. Low is 2200PSI. High is 3000PSI.
Both are filled in the back room of dive shops. They put them into a tub of water to cool them.
The Honda Mirai runs the 700 BAR version that, when full, carries 5 kilos (about 10lbs) of hydrogen.
The last time I looked up the specs on those tanks, they were carbon fiber reinforced plastic and weighed in at a bit over 300lbs for the pair of tanks used. The fuel cell is about 100lbs.
Compare that to the weight of the Tesla battery pack at 1400lbs.
The electric motors in the cars don’t care where they get electricity from so that’s kind of a wash in terms of weight comparisons.
If the price is right, I think this would be a good addition to homes; as back up power supply or maybe the primary power supply. Too early to to project that far, but my thought is that a closed small scale house unit (placed outside the house, similar to current emergency power backup units) with potential recycling capabilities with feed port for needed raw material supply.
Mark, it would have to be practically free to be more cost effective than batteries, as a relative energy loss of from 29% to 51% is a daily loss, not just a startup cost. Here is a quote” “The round trip efficiency of energy storage in batteries as shown in Table 10.3 is in the range between 70% and 95%, while in the case of a hydrogen system using a 350 bar compressed gas storage, one can expect a round trip efficiency of only 47% [2]. If one allows for the use of heat released during the conversion of hydrogen back to electricity in a fuel cell on site, the overall energy efficiency can be increased to 66%” from sciencedirect: https://www.sciencedirect.com/topics/engineering/round-trip-efficiency#:~:text=The%20round%20trip%20efficiency%20of,only%2047%25%20%5B2%5D.