Renewable energy sources are becoming increasingly popular. However, such energy can be wasted if an excess is available when it’s not yet needed. A particularly relevant example is solar power; solar panels provide most of their output during the day, while often a household’s greatest energy use is at night.
One way to get around this problem is by storing excess energy so that it can be used later. The most common way this is done is with large batteries, however, it’s not the only game in town. Phase change materials are proving to be a useful tool to store excess energy and recover it later – storing energy not as electricity, but as heat. Let’s take a look at how the technology works, and some of its most useful applications.
It’s All About Heat
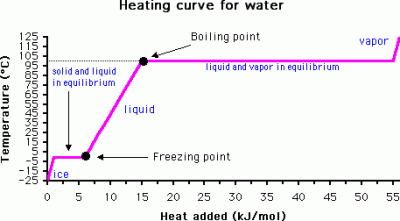
Unlike batteries or capacitors, phase change materials don’t store energy as electricity, but heat. This is done by using the unique physical properties of phase changes – in the case of a material transitioning between solid and liquid phases, or liquid and gas. When heat energy is applied to a material, such as water, the temperature increases. However, when the liquid water reaches temperatures close to boiling point, something strange happens.
As more energy is put in, the temperature begins to flatline. This is because enough energy must be put in to overcome what is called the latent heat of vaporization – the energy required to turn the liquid into a gas. Eventually, once enough heat is put in, the water turns to steam and the temperature is again free to rise. This latent heat can store a significant amount of energy in a material over a relatively small temperature change. This latent heat exists in solid-to-liquid phase changes as well, where it’s known as the latent heat of fusion. By taking advantage of latent heat, large amounts of energy can be stored in a relatively small change in actual temperature, and accessed by manipulating the phase change of a material.
Perhaps the most common form of phase change heat storage on the market is the sodium-acetate handwarmer. These handwarmers contain a sodium-acetate gel in a plastic pouch. When the gel is given a nucleation point by tweaking a metal disk in the gel, it quickly changes phase from a super-saturated liquid to a solid. Suddenly freezing like this releases the latent heat the material was holding in its liquid form, and warms the user’s hands nicely. The material can later be recharged by heating the handwarmer up to melt the sodium acetate once more, before allowing it to gently cool back down to room temperature. The latent heat will then be trapped in the liquid until it is once more disturbed, causing it to freeze again.
A wide variety of materials have been studied for heat storage through the phase change effect. Paraffin wax is perhaps one of the most commonly studied, thanks to its phase change occuring in a useful temperature range. However, its low thermal conductivity limits the rate at which energy can be exchanged, hampering performance. Hydrated salts have been another material of significant interest, though face problems of their own. Often, such materials will undergo subcooling. As heat is extracted from the liquid material, its temperature declines below freezing point without the material actually becoming solid. Without undergoing a change in phase, the latent heat remains trapped in the liquid, and can’t be extracted. Additionally, like many battery chemistries, repeated cycling can cause problems. The phase change material must retain its properties over many cycles, without chemicals falling out of solution or corrosion harming the material or its enclosure over time. Much research into phase change energy storage is centered around refining solutions and using additives and other techniques to engineer around these basic challenges. Often, the specifics of such materials remains a commercial secret as companies attempt to recoup research costs through sales.

The phase change effect can be used in a variety of ways to functionally store and save energy. Heat can be applied to a phase-change material, melting it and thus storing energy within it as latent heat. Excess electrical energy, such as from renewable sources, can readily be stored in such phase change materials, as it’s possible to turn electrical energy into heat quite efficiently. The reverse is not so easy, however.
Instead, such phase change devices are often instead used to output heat more directly – either by being used as hot water heaters or to supply heat energy to refrigeration processes. This is achieved often by simply passing working fluid, like water or refrigerant, through a heat exchanger in contact with the phase change material. The former has plenty of applicability to households, cutting down on costs for residential heating and hot water. The latter is of more relevance to large commercial and industrial facilities. Particularly in industries such as winemaking and cold storage, refrigeration can be a major bottom-line expense that is essential to operations. Even small percentage gains in efficiency or reduced energy use can have huge payoffs over time.

Another interesting use of phase-change materials is as a passive heat management solution for buildings. The idea is to use a phase change material with a melting point around a comfortable room temperature – such as 20-25 degrees Celsius. The material is encapsulated in plastic matting, and can be installed in a building in walls and ceilings along with insulation. The material then acts as a sort of thermal buffer. Heat energy building up in a room can be absorbed by the phase change material, keeping temperatures lower. As the building then cools, the material can release its heat, acting to stabilize temperatures. It can be a lightweight way to increase the thermal mass of a building, and can reduce the reliance on active cooling or heating from HVAC systems.

Looking to the future, it may be that phase change energy storage remains of limited use in the residential space. While it can have benefits, its limited heating-only application makes it less attractive than battery storage that can run an entire home. However, for industrial processes, such as refrigeration and process heating, there’s plenty of scope for phase change technologies to be used as a cheap and effective store of energy. With research ongoing in the field, it’s likely we’ll see greater uptake of this technology in future as energy conservation increases in relevance in future years.

















We just need to find some of that special water they had in “Steam Boy” (Allowing super high pressure steam to be stored for a long time)
It’s called hydrogen peroxide, H2O2. Used for jetpacks.
Personal flight technology has forked into two broad fields, with further forks sure to come. The traditional hydrogen peroxide monopropellant devices are know called Rocket Belts, while the Jetpack term is now more commonly used for gas turbine devices burning kerosene with atmospheric oxygen. I wonder what they will call devices that hybridize the two technologies…
Also in VW beetles apparently, ;-)
https://www.youtube.com/watch?v=kpg4g47ssFY
I like the demonstration of how safe it is – H2O2 and methanol can’t be ignited by fire!
…as long as the fire is small enough that the hydrogen peroxide doesn’t start to decompose by the heat alone. If it does, the methanol starts to react with the pure oxygen and release more heat, and you have a runaway reaction that ends with an explosion. Also, organic material like the oil from your fingers act as a catalyst for the hydrogen peroxide, which is why the guy wipes the mixing stick with tissue paper.
The video is like watching someone play with a stick of dynamite, banging it against the edge of a table going “Look how safe this is!”.
haha, you are so right. If that is 50% H2O2 it is some seriously dangerous stuff. I use 35% H2O2 that I modify into Peroxone for some experimental rocketry. At 35% it will rapidly decompose, releasing a ton of O2, from contact with a very wide variety of chemicals or contaminants.
That surge of Oxygen turns a wide variety of things you would never expect into highly combustible substances.
I would never consider using 50% H2O2 in a car ever. In even a moderate crash it would turn the vehicle into a highly oxygenated fireball deathtrap.
That was common in the 1800s. A steam accumulator is just a well insulated unheated boiler partially filled with high temperature water. Adding steam charges the accumulator, with an increase in temperature, while drawing off steam to power equipment removes heat with the steam. It seems evident that it won’t supply superheated steam.
It is in fact a phase change energy accumulator, typically operating in the 100C to 300C range.
Don Sadoway at MIT invented a form of liquid metal battery that does this, that stores and releases electrical energy.
In addition to the solid/liquid phase change, there’s also an alloy/elemental phase change going on. There’s interesting chemistry as well – the two liquid metal electrodes form an alloy, not a compound. (Sort of…) Storage efficiency and energy capacity are pretty high, although the cells are too heavy for any mobile application.
https://en.wikipedia.org/wiki/Molten-salt_battery#Liquid-metal_batteries
Ambri has started making and shipping batteries based on his design: https://ambri.com/technology/
Well there’s the idea of using ice storage for cooling.
https://en.wikipedia.org/wiki/Ice_storage_air_conditioning
And the idea should be reversible for the winter months.
If you want to have a ground-source heat pump without the cost of sinking miles of tube under your yard, just draw cold water from the tap and put it in the freezer, then keep chucking the ice cubes out the door.
You know… Your right
Interesting… I have my own well and a freezer with a connected ice maker. All that’s left is to build an ice chute with an insulated flap to the outside, and a gizmo to poke the ice maker and open the flap at intervals :) The water from the melting ice could be directed back into the well. It’s probably gonna take a while to heat up the house that way though… Maybe it’s time to put that rain water leak in the basement to use and go shopping for an industrial size ice maker :D
It’s a serious proposition though. Houses lose tremendous amounts of heat down the sink.
Imagine if you had a pool of water underneath the house for a holding tank for all the grey water that’s going down the sewers. Even cold tap water is 14-15 C and if you run a heat pump, you can extract 4.19 kJ per kg per C. Taking a cubic meter of cold tap water down to 1 C with a heat pump would gain you about 16 kWh of heat.
The average US household uses about half a cubic meter of water a day, and a great deal of that has been already heated (shower, dishes, clothes washer), which means the recoverable amount of heat is probably enough to sustain the temperature of the house without any additional heating.
In practice, to avoid the pool of stagnant sewage, you would have the opposite of a hot water heater: a reservoir of water (or sand etc.) that is kept close to 0 C by the heat pump that heats up your house, and all the sewage passes through a loop of pipe inside that reservoir so that the exit temperature is close to freezing.
In the summer, you can use it in reverse to dump you AC heat, so having a cold shower has the effect of cooling down the house.
Yep, even though I was joking a bit in my comment, I do see the potential. You could even store ice from the winter in an insulated holding tank to reuse for AC or even direct air cooling during the summer. Put an ozone generator in the tank to keep microbial growth at bay maybe. Separate the not so grey water (shower, laundry, probably were most heat loss is in my case) and you might be able to run that through a traditional heat exchanger directly and not have to deal with poorly conducting sewage pipe.
I’m seriously looking into this but need to do some math to see if it’s feasible (how big is that heap of ice going to get for starters? How do you minimize losses when the ice needs to be released?). My air-to-air heat pump needs replacement anyway. The catch for me might be that I have no radiators and no liquid side heat pump, so the initial expense will be pretty big unless I can cobble together something with an indoor air unit and a cheapish “outdoor” liquid exchanger. The reverse is pretty common off the shelf, but that doesn’t really help…
I’m surprised boron was not mentioned. I read an interesting article about boron-based batteries, where that could be “recharged” simply by removing the solid boron and replacing it with a liquid.
But it has an inconveniently high melting point of ~2000°C or so…
That’s where LASERS come in handy! Pew! Pew! Pew!
There is also the use of excess energey to liquify air that can be subsequently used to power a turbine generator; not exactly a domestic solution but phase change storage nevertheless.
There isn’t such thing as thermal mass. What could it be ? gramKelvin ?
If you were to make an analogy, don’t refer to mass but to capacitance instead, since it’s what you meant, I think.
Pedantry against the commonly accepted terminology, especially accepted in technical contexts, is unhelpful
Thermal mass is common speak for specific heat capacity – Joules/(Kelvin.Kg) – a measure of energy stored/cost of changing the temperature of a mass of material x.. So its a perfectly valid description..
So in this case because of the phase change you have a very high specific heat energy for very low actual mass.. Which is pretty cool, usually in buildings you fill with concrete/block/bricks or have lots of water around to get thermal mass – largely by having large mass..
Thermal mass is heat capacity – as in, *not* per unit mass, or just straight joules/kelvin. Think about it : if you say “yeah, the ground plane’s got a ton of thermal mass, so it’ll take longer to heat up,” it’s because the ground plane just has more copper than other signals do.
The “fancy term” would just be heat capacity (no ‘specific’, which indicates that it’s an intensive property, rather than an extensive property – like specific impulse, or how ‘density’ could be called ‘specific mass’), but it’s the same as using “voltage” instead of “potential difference” – whiny pedantic types lost out to people who actually do the stuff.
The nature of the language is such that like ‘strength’ being a term that could mean stiffness, yield force, tensile or compressive etc thermal mass is the simple speak term commonly used without a great deal of precision for anything loosely related to the specific heat capacity. So like rather a lot of things in English its very tied to the context as to what it really means…
So while you are right, and I’m sure your English teacher would be very proud, unfortunately in the real world we just don’t talk with that precision, and usually I expect wouldn’t actually be understood if we tried to…
I don’t know why you think this is a “language” discussion. No one uses “thermal mass” to mean “specific heat capacity.” A drop of rain has a higher specific heat capacity than a block of concrete, but no one would say “yeah, that drop of rain’s got a ton more thermal mass than that block of concrete.”
It’s just heat capacity. This isn’t complicated. It’s a common term in building design, and physics and engineering in general. It’s also worth noting that having a high specific heat capacity doesn’t necessarily lead to a high thermal mass for a component in a building, either: if material A has high specific heat capacity (lowercase-c) and low density, it won’t necessarily be better for a building than material B which has a lower specific heat capacity and higher density, because the constraint in a building is mainly by volume.
“Thermal mass” obviously refers to the mass that is storing heat, which implies the total capacity and not the specific capacity.
It is not common to think in terms of specific capacities, because things come in definite sizes. This is not a question of language, but a property of practical reality.
Actually Pat round here at least they frequently do, if you’d calculate it using the specific heat capacity of the involved materials doesn’t matter if you are adding more mass or using a different material ‘thermal mass’ is often used as the catch all, context required to make sense of term. Often though not always as a direct replacement for specific heat capacity (presumably because the non science/engineer types have no idea what that term means, but can with context know exactly what it is is if you use friendly simple common words)
“There isn’t such thing as thermal mass. What could it be ? gramKelvin ?”
Joule/kelvin. Thermal mass is specific heat * mass. And yes, “thermal mass” is the right casual term. It’s resistance to a change in temperature, just like mass is a resistance to acceleration.
If you’re going to get pedantic about casual terminology, you’ve got a long fight ahead of you. Similar pedantry has tried to get rid of amperage, voltage, tonnage, grammage, and mileage. You can see how well those battles went.
“hot water heater” is an oxymoron people, if the water is hot, why on earth heat it ?
… obviously to make it hotter ;-)
Wouldn’t that be a boile?
Yes it would be called a boiler, and it is called that.
But more on the technical point of it, the hot water tank should never stay below 55 C because that permits bacteria to grow in the pipes, which risks legionnaire’s disease, so a hot water heater IS literally a hot water heater insofar as it should always remain hot.
How it works is by passing cold water from the mains through a tube inside the hot water heater, which in turn makes the cold water hot. You never consume the water inside the hot water heater.
water heaters have a pressure relieve valve, should the contacts on the thermostat weld together, makeing the heater a high pressure bomb , unless you’re makeing a steam generating turbine , then you have Steam
The salts-based passive temperature modulation systems were popular in retrofitted “solar” (often sunroom – convection based) installations that I saw in the 1980’s and a few are likely still in service despite their need for occasional messy servicing. When they worked well it was an extraordinary thing to be in a house that simply stayed warm without a roaring furnace or anything else going on. Ice storage experiments have come and gone, usually doomed by variability in winter weather, though the more prosaic geothermal systems as heat source/sinks are in everyday service.
The article might make the distinction between storing/retrieving thermal energy in general and creating the electricity that HAD is so fond of manipulation – the jump from one to the next took a good deal of time in the Industrial Revolution, and will likely be a bit more finicky for your Tesla.
Do a double heat exchanger on a solar thermal heater, First heat up some type of oil (mineral, corn , used motor oil something that can do high temps without breaking down.) Store in a well insulated container then run water inside the second exchanger and the steam that is made can be run through a steam engine that turns a generator for power. No salt used because that is a mess to maintain.
Cold Fusion