We’ve all been there: you’re sitting at your bench, with a beaker full of some conductive fluid with a bunch of tiny particles suspended in it, and you want to measure the sizes of each particle.
Okay, maybe this isn’t a shared experience we’ve all had, but It’s at least an ordeal Hackaday alum [Nava Whiteford] has been through, and he was able to carry out the measurements in question using a neat apparatus known as a Coulter counter.
Imagine a container full of a conductive fluid. If you place an electrode at each end, the fluid will carry a current.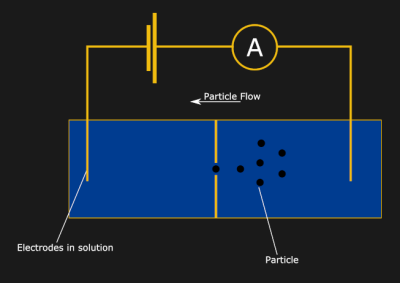 Now, drop an insulating divider in the middle of the container, and the current will stop flowing. Finally, poke a small hole (or nanopore) in the divider. Huzzah! The current is flowing again… but how does this let us measure particle sizes? Well, now think about a tiny particle moving through the hole in the divider. As the particle passes through, the hole will be partially blocked, and the current flow will be partially interrupted. It turns out, the resulting dip in current is proportional to the volume of the particle — a fun property known as the Coulter principle.
Now, drop an insulating divider in the middle of the container, and the current will stop flowing. Finally, poke a small hole (or nanopore) in the divider. Huzzah! The current is flowing again… but how does this let us measure particle sizes? Well, now think about a tiny particle moving through the hole in the divider. As the particle passes through, the hole will be partially blocked, and the current flow will be partially interrupted. It turns out, the resulting dip in current is proportional to the volume of the particle — a fun property known as the Coulter principle.
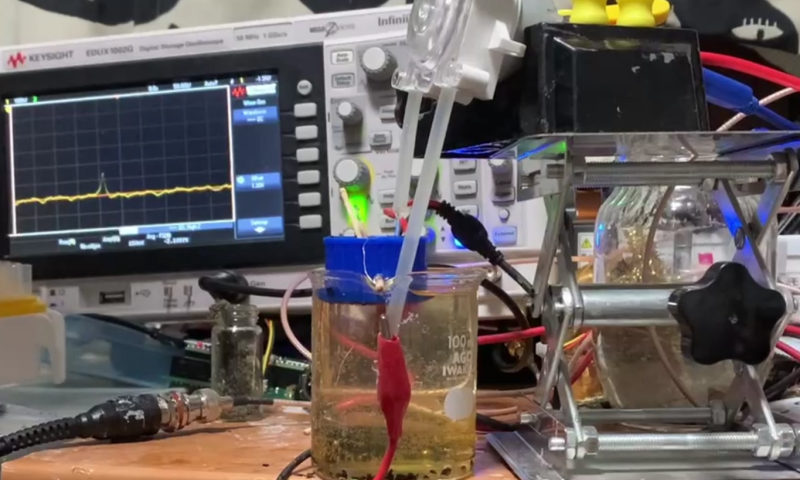
[Nava] built a great demo of the system with a macropore in place of the nanopore. The pore in question was a hole melted into a bottle cap, which was suspended in a beaker by two toothpicks. [Nava] used small chips of Acrylic as the particles to be measured, which they pipetted into the solution of KCl. They then passed a current through the solution and used an oscilloscope to sense the interruptions. Be sure to check out their write up for a video of the system in action!
Of course, this technique has a much wider range of applications than measuring little bits of plastic — obtaining blood cell counts, for one. We’ve seen particle counters for use in the air before, but it’s great to see that there’s a way to measure particles in an aqueous solution — you know, in case we ever find ourselves in such a situation.















Doesn’t it depends on the particles being somehow ionized/charged? Otherwise they would not be forced through the opening?
Not necessarily, because the KCl will cause a hydrodynamic current and drag neutral particles along
In this case, the hydrostatic pressure across the pore is probably what’s driving particles through. If you pipette liquid into the bottle cap, it has to travel through the hole, to equilibriate the fluid height with the fluid outside the bottle cap. Some particles get carried through by the flowing liquid and bam – resistive pulses you can measure with a transimpedance amplifier.
Your intuition isn’t wrong, though. The other two forces that are typically at play in these systems are electrical – electrophoresis of the particles if they have a surface charge, and electroosmosis through the pore if the pore has a surface charge. The particles don’t need to be ionized; rather ions in solution will usually just adsorb chemically onto the particle surface and it’ll acquire a charge that way (look up Electrical Double-Layer theory for more specifics).
The Coulter Counter has been used in medical laboratories for over 60 years to perform CBC’s Complete Blood Counts, a very common hematology test that nearly everybody has had at least one time in their lives.
I worked for Beckman Coulter, the namesake of two true giants of laboratory analytical development. Arnold O. Beckman and Wallace H. Coulter.
For those with a university library subscription and a desire to read more, a lot of the literature on this uses the term ‘Resistive Pulse Sensing’ interchangably with the Coulter principle – particularly when talking about micro/nano-scale particle sensing.
This is how the nanopore sensing devices from TwoPoreGuys, later Ontera, worked. The Teensy 3 based device sensed picoamp current dips as a DNA scaffolding was sucked through the nanopore, either loaded with an assay target molecule, or empty.
It took a while to get a super sensitive amp that didn’t get swamped with noise.