We know, we know. Generally speaking, you should try and switch your household devices over to rechargeable cells rather than using disposable alkaline batteries. But while they might seem increasingly quaint in the lithium-ion era, features such as a long shelf life make it worth keeping a pack of disposables around. So which ones should you buy? That’s what [Moragor] wanted to find out with his personal battery analyzer.
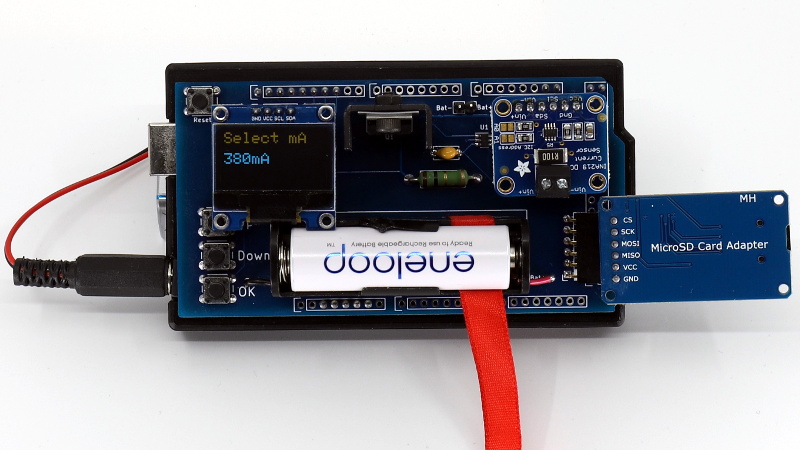
Designed as a shield for the Arduino Mega 2560, the analyzer combines a small programmable electronic load with a INA219 current sensor, OLED display, and SD card reader. The user selects the cutoff voltage and discharge rate before the test begins, and once it’s running, data is collected every second and saved to the SD card for later analysis. Once the battery voltage reaches the predetermined value, the test is over and you’re ready to put a new cell through its paces.
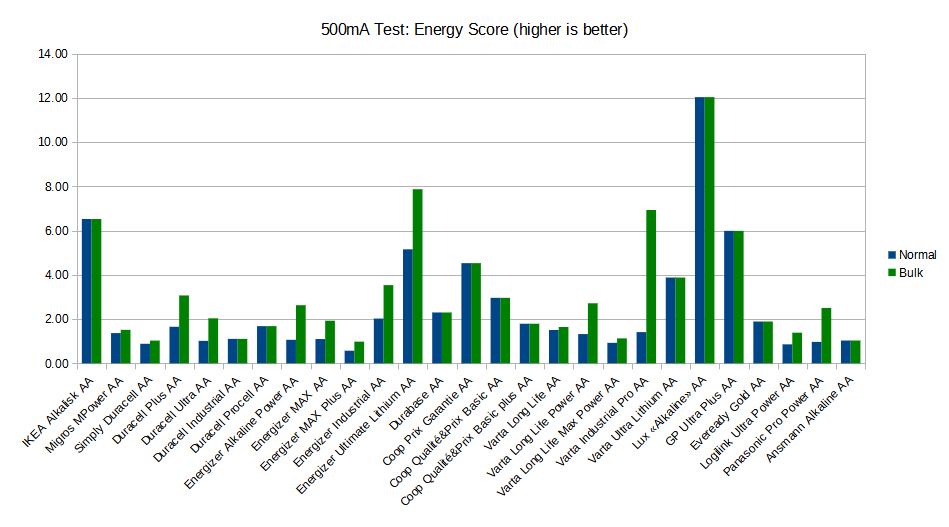
After testing 27 different brands of batteries, [Moragor] tabulated all the data and produced some helpful charts to illustrate the results. With few exceptions, the performance level for most of the batteries was remarkably similar. If anything, the test seemed to show that higher tier batteries from companies like Duracell and Energizer actually performed slightly worse than the mid-range offerings. Perhaps the biggest surprise is that, when the per-cell cost was factored in, the local cheapo batteries provided a better value than anything else in the test.
While the selection of battery brands may be different from where you live, the data [Moragor] collected is still a fascinating even if you don’t recognize some of the names on the chart. Of particular note is the confirmation that lithium batteries handily outperformed any of the Alkaline cells tested when it came to high-drain applications. We’d still rather they came in rechargeable form, but at least it’s a step in the right direction.















Another nice resource, albeit a bit old, is https://lygte-info.dk/review/batteries2012/CommonAAcomparator.php
Lucky for me, Moragor seems to be swiss as well: the own Migros and Coop-Brands are also tested. Noice! :)
Seems like i stick with my IKEA batteries…
Also noteworthy: IKEA LADDA 2450 are in fact relabeled Eneloop Pro. IKEA is MUCH cheaper than the ones with the black sticker (like factor 5 or so).
I have used the eneloop and eneloop pro for many many years now. Having tested the LADDAs as well I can tell you of my findings:
Using an AV4m charger I never had a 4 pack of Eneloop being off for more than 4…5mAh to the other ones.
The LADDAs are a bit more mixed and have higher tolerances capacity wise. I think they ARE Eneloops but are not the super tightly matched ones but the 2nd class batches.
So, if you need very tightly matched cells for packs or situations you need 8 of them in séries or so, the Eneloops still seem better…
ive noticed it too, but i only noticed the preformance.
before trying IKEA-LADDA, eneloop was the only “good” NiMh AAs i had ever used, second to Duracell NiMh AAs, which i havent tried in years.
due to me having never tried the eneloop-PROs, i see the IKEA-LADDA as best.
after trying IKEA-LADDA, i stopped buying anything else until corona hit, now im limited to grocery-store rechargeables aka energizer… maybe someday i can try genuine eneloop-PROs.
@Thomas said: “…IKEA LADDA 2450 are in fact relabeled Eneloop Pro. IKEA is MUCH cheaper than the ones with the black sticker (like factor 5 or so).”
But it looks like the LADDA 2450 are going away:
“LADDA – Rechargeable batteryHR6 AA 1.2V – $6.99/4 pack – Last chance to buy”
https://www.ikea.com/us/en/p/ladda-rechargeable-battery-70303876/
Duracell is looking shadier and shadier these days. If you find leaky batteries in a device these days, it’s more likely to be a Duracell than anything else. And the capacity is the worst in the above list. I guess they’ve just decided their market position is strong enough that there’s no downside to sourcing the cheapest, poorest cells they can find.
Marketing cost a lot.! Must come from somewhere.
I second that! Duracell ALWAYS leak. They were responsible for 100% of the electronics I have seen damaged by cell leakage over the last 20 years.
Third-ed
4thed. I’ve stopped buying them completely.
Me too.
The first time it happened to me, I thought someone must have sold me a pack of counterfeits. I’ve since come to realize that leaking and destroying devices is just what Duracells do now.
I recently had a whole brand new pack of Duracells pop and start leaking. Dated 2028 or something like that. Add that to all the electronics I’ve lost over time to them.
Never again.
Current drawer has some Rayovacs in it, but based on this I might just start going to Ikea.
Duracell went down the drain after the Proctor and Gable acquisition of Gillette a number of years ago. They ran the operations nearly into the ground before offloading the brand to Berkshire Hathaway. I stubbornly still buy them because I know some of the people that the AA / AAA production supports.
Wish RayOVac was in the test. Quit Duracells due to leakage, but also had to stop using Ikea due to leakage. Don’t see it with RayOVacs, but no idea if their energy is comparable.
>you should try and switch your household devices over to rechargeable cells
Alkalines are still better for low drain applications or intermittent operation. There’s two things going for it: the energy density gets dramatically better at low currents and pulsed discharge, and it doesn’t present a fire or chemical risk when fully discharged. The main competitor is Lithium Thionyl, but it has the problem where you have to “wake” the battery up before it will give much current, and it’s quite explosive when mistreated.
One interesting place to find alkaline batteries is paper towel dispensers or more recently portable hand sanitizer dispensers, which quite often have a row of D-cells inside to run the motors. You could use rechargeable lithium cells there, but then you have to spend working hours carrying them back and recharging them, measuring them, tracking their age and condition, generally taking care of them, which requires more expertise and time – and most importantly the storage and working facilities – than your typical roaming cleaning worker doesn’t have, which makes it cheaper and simpler to use disposable cells.
If mass is not an issue, then alkalines come cheaper, because you skip the whole trouble of maintaining the batteries back in the shop. Especially if the device is a handheld meter of some kind which might be sitting on a shelf for three years before it’s needed the next time – by which time the lithium or other batteries will have gone bad out of disuse and may fail you in the field, and in any case you can’t just pick it up and slot in new batteries because it needs recharging first.
There’s really nothing wrong with alkalines. They’re cheap, non-toxic, safe, easy – you can dispose of them with regular household waste because it’s typically just zinc and some mild lye in there. The only issue is that they’re so cheap to make it isn’t economical to recycle them, which wastes the materials into landfill – which is a problem with all the alternatives as well. Only about 5% of lithium cells get recycled because it’s cheaper to make new ones.
Quite, so for real low-drain applications (e.g. thermostatic radiator valves) I would also be wanting to see a measurement of self-discharge rate.
My last set lasted for 3 years, so that becomes a significant factor.
For applications with low and steady discharge, lithium thionyl is the best – you can get gargantuan amounts of energy in a single cell that will last at least 10 years – but you have to have a low-voltage cutoff circuit or else bad things will happen.
But, if it’s a device that needs to provide some amount of pulsed output current, like turning a motor or something, then the thionyl cell fails because it can’t do high current output.
You’re not recycling batteries?!?
https://brussels-express.eu/belgium-is-top-european-country-in-battery-recycling/
Collection of used small format batteries is patchy at best, and recycling thereof is largely absent. Many times I’ve seen, nobody empties the collection boxes or they’re just dumped with the rest of the trash because nobody is accepting them.
>European average (44%).
The local (government run) recycling center does not accept alkaline cells, they tell us to throw them in the regular trash.
The material costs of an AA battery is less than a couple cents. The handling, refining and transportation costs exceed the value of the reclaimed metals, which means that you can cause more environmental damage by recycling them than not (other energy/material use).
Moreover, when municipal waste is sent to incinerators, the metals are removed by magnets and eddy current separators which throw things like bits of zinc and aluminum off the conveyors. This collects the used batteries anyhow.
Thanks for the reply, Dude!
And the additional issue is that there’s no shortage of zinc. It comes as a side product of lead, silver, copper, molybdenum mining, so even if recycling was cheaper it would simply crash the market price and make recycling uneconomical again.
A great deal of the recycling we do is either not recycling (actually down-cycling) or it’s pointless, and only done because it’s subsidized and/or forced by regulation.
> The main competitor is Lithium Thionyl, but it has the problem where you have to “wake” the battery up before it will give much current, and it’s quite explosive when mistreated.
Do you have any resources where I could find out more about this? I mean, what maximum pulsed current (10ms) could I get out of alkaline battery?
See manufacturer datasheets for Lithium thionyl cells. For example, a Varta type number DS7106 lithium thionyl chloride cell has 3.6 V and 2,500 mAh, maximum continuous discharge 60 mA, maximum pulsed current 150 mA for 100 ms every 2 minutes.
The reason why lithium thionyl gets such a great shelf life is because it forms a passivation layer on the electrodes, which protects it from self-discharge in storage. It requires a minimum discharge current to be maintained or else the voltage sags the next time the device draws current – though you can avoid that by pulsing the battery occasionally.
https://www.jauch.com/blog/en/passivation-lithium-thionyl-chloride-batteries/
I just measured roughly 1 Volt and 2 Amps into a 0.5 Ohm load from a single no-brand alkaline AAA size cell. Since the open cell voltage was roughly 1.6 Volts, that’s a respectable 0.3 Ohms for the internal resistance. About 0.2-0.3 Ohms seems to be common for AA/AAA sizes. The voltage output was flat on the oscilloscope – there’s no discernible “capacitor effect” like with the older zinc-carbon cells. In general, you can expect at least 10x the pulsed current output from an alkaline.
That’s the most useful and straightforward information I’ve seen today! Thank you very much!
A major advantage of lithium thionyl though is that the discharge voltage curve is absolutely flat. It just keeps on going until it stops, which is why it’s used for memory backup cells. You don’t need any regulators in between, which would leak current needlessly.
oh I’m surprised how good the IKEA cell are
I bet it comes with a simple instruction how to put the battery together.
Or horse meat.
What is wrong with eating horse meat?
and there will be some screws left over!
I got this odd little hex wrench with mine. Any ideas what that’s about?
Dont be, they also sell excellent cheap LED bulbs, it all comes down to strict quality control of their sources.
Well, I am planning to buy some (online, no store near me).
But I better hurry!
https://www.ikea.com/us/en/this-is-ikea/newsroom/ikea-to-remove-non-rechargeable-alkaline-batteries-by-2021-pub6065b612
That Ikea link inadvertently reveals the dirty truth: The win you get by using rechargables is so vanishingly marginal that you have to babysit a rechargeable through FIFTY charge cycles to make it more environmentally “responsible” than a disposable.
The question of which to use is pretty much moot in most cases. Low drain, long standby life = use single-use ones. High power = use rechargeables.
Now, if we can just get designers to get the quiescent drain of devices down so the shelf life actually usable. I’m tired of pulling seldom-used meter out of the drawer to find the battery dead because the designer was too lazy to get the standby drain down to a few uA. I’ve added hard power switches to a few of them because of that.
The edge case is long standby AND high power, such as in emergency flashlights, alarms and sirens, and indeed meters and devices that are put on the shelf and rarely used. Lithium rechargeables have low enough self-discharge, but you can’t leave them in fully charged because they get bad shelf life like that, which defeats the point.
The photo shows an Eneloop battery. That’s NiMH. It’s not on the chart and rechargeables are not mentioned anywhere. What about rechargeables?
It’s article about hackaday.io project, which started with testing NiMH, but author also tested some alkaline batteries. Please read first link.
Eneloops original “deal” was they were meant to have low self discharge, so performed more like an alkaline (Than regular NiMh) in low drain applications.
The device is obviously capable of testing rechargeable batteries, but the goal of the project was to test alkalines.
Also the lithium AA battery is lighter and wont leak and good in low temp enviroment like temperature sensors. Good for camping/hicking devices.
Totally. The lighter weight and better performance of lithium compatible batteries is noticeable on caving lamps/climbing. And while rechargeables are great, having a box full of spare, reliable energy for your light, when your most of a day away from an entrance of a cave is a great thing.
Primary cell lithiums won’t leak by corrosion, but if damaged otherwise they can do nasty stuff.
The typical alkaline replacement lithium cell is lithium-iron-sulfite with flammable solvents in the electrolyte (Propylene carbonate, Dioxolane, Dimethoxyethane). People sometimes use a knife point to dig the batteries out of devices, with bad results.
These are even more fun:
https://uk.rs-online.com/web/p/aa-batteries/5268431
Lithium Thionyl Chloride batteries don’t like being charged. Most impressive kaboom from a 1/2 AA size.
I recently found an old remote I haven’t used in over 12 years with Duracell Powercheck? batteries inside (with ca 25-35% charge according to the indicator). I was surprised not to find the typical battery leaks I have become used to finding ie. in flashlights not used in a while. I guess aging and durability are further factors to consider for certain applications.
What about variations between batterys made in Asia, the US, and Europe?
I’m mystified by the circuit. I would expect that opamp to be unhappy about driving that honkin’ great capacitive load C2, and so I’m unclear why it’s even there. If for stability, I would expect connecting the cap to the negative input instead of ground would be the way. (or put a resistor in series with the opamp output)
Out of interest I checked the datasheet for the tlv2372, for having a cap on the output:
> 8.3.2 Drivinga Capacitive Load
> When the amplifier is configured in this manner, capacitive loading directly on the output decreases the device phase margin leading to highfrequency ringing or oscillations.Therefore, for capacitive loads of greater than 10 pF, TI recommends that a resistor be placed in series(RNULL) with the output of the amplifier ,as shown in Figure34. A minimum value of 20Ω should work well for most applications.
https://www.ti.com/lit/ds/symlink/tlv2372.pdf
Yes, the reason why it’s there is stability. I’m not very experienced in electronics, so a few oopsies in the circuit are to be expected. But hey I just learned something new and if I ever build a MkIII version that will be fixed.
My concerns are less around specific brands than finding things that aren’t flat out counterfeits or otherwise obviously fraudulent.
Finding legit CR2032 batteries as well as various button cell sizes is a real challenge. In the button cell sizes, you’ll get significant portion of the batteries already defective – if they bulged, they’re done for and another percentage will fail long before use so maybe you get about 25% that work reasonably well. I see batteries with the name in the wrong font, misspelled, or in the case of higher capacity rechargeables like the 18650, with insane listed capacities.
It’s a real bummer…
I’ve switched to ordering CR2032 only from digikey (added to some order with other electronics) and it has made a huge difference to the longevity. The prices are also good as long as you don’t compare with the crap on amazon… (I’m sure the other electronics distributors also have genuine cells)
I’ve had huge failure rates 50% or worse trying to get name brand lithium coins/buttons in electronics and big box stores. I think most of them don’t get the turnover on those and it’s stale stock… even though in theory they should be good for a decade. Buying from the dollar stores, I seem to get better than 20% failure rates and 80% good enough.
I say or worse because of only getting 2 or 3 months in a watch where the original battery did 5 years, appearing “good” for long enough you can’t take it back, but being far far short of reasonable performance. I’ve got dollar store batteries going 2 or 3 years in the same watches.
In reqards to the Energizer Lithium cells.
I keep a spare set of those for my DSLR.
The DSLR won’t even work with alkaline cells.
Project Farm on youtube did a fairly credible review of rechargable AAs. He may have at some time done a similar review for non-rechargables…
Not really. I built the analyzer to test some NiMh batteries that seemed to be quite bad and then started to test various NiMh brands. These tests are still going because I also test the long time self discharge. I started testing Alkaline batteries out of curiosity and because its kinda addicting :D
I’m puzzled how the data on the chart is ordered.
For the Australian readers, Gough Lui did an excellent test of all the batteries on the Australian market: https://goughlui.com/2016/12/19/great-aa-alkaline-battery-test-pt-1-battery-testing-fundamentals/
TLDR: Varta batteries from Bunnings FTW.
Ha Ha,
Very happy to see this type of study. In 2014-2015 I have done this type of testing and am happy to see that Tom comes to the same conclusions as me : https://synthelectro-fr.blogspot.com/search/label/E-Piles-Batteries (In French, sorry).