As ubiquitous as rubber tires are due to the many practical benefits they offer to cars, trucks, and other conveyances, they do come with a limited lifespan. Over time, the part of the tire that contacts the road surface wears away, until a tire replacement is necessitated. Perhaps unsurprisingly, the material that wears away does not magically vanish, but ends up in the environment.
Because of the materials used to create tires, this worn away material is counted as a microplastic, which is a known environmental pollutant. In addition, more recently it’s been found that one additive commonly found in tires, called 6PPD, is highly toxic to certain species of fish and other marine life.
There are also indications that these fine bits of worn-off tire contribute to PM2.5 particulate matter. This size of particulates is fine enough to penetrate deep into the lungs of humans and other animals, where they can cause health issues and exacerbate COPD and similar conditions. These discoveries raise a lot of questions about our use of tires, along with the question of whether electric vehicles stand to make this issue even worse.
A Lot Of Dust
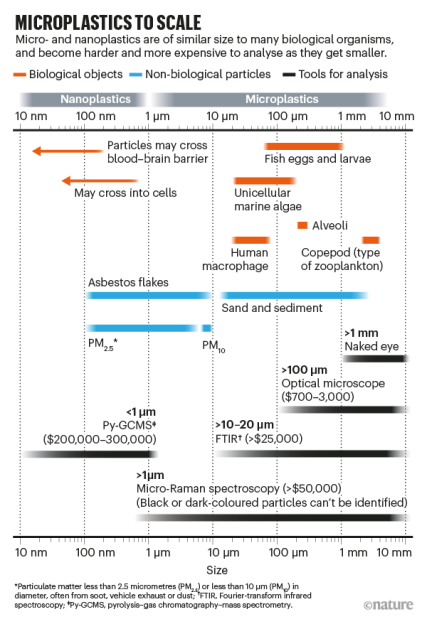
An obvious question that comes to mind when confronted with this issue is just how much material we are talking about. Kole et al. (2017) published a study that answers exactly this question using a collection of statistics and educated extrapolations. Their estimate comes down to between 0.23 to 4.7 kg/year, with a global average of 0.81 kg/year/capita. The estimated contribution of tires to the microplastics that end up in oceans annually would be 5 – 10%.
Most notable about these numbers is perhaps that this means that the particulate matter (PM2.5) pollution produced by the wear on tires is significantly higher than that produced by the tail pipes of internal combustion engine vehicles (ICEs). After decades of regulating the exhaust gases from combustion engines, it would seem that it is now time to look at other sources of this type of pollution. Especially since this type of particulate matter is not one that will vanish as vehicles switch from ICEs to batteries and electric motors, as the latter will still have tires.
An awkwardly situated elephant in the room that further complicates this matter is that the weight of BEVs tend to be higher than that of ICE cars due to the weight of the battery pack. This may lead to BEVs wearing down tires faster and thus creating more microplastics. Whether this will this turn out to be an issue remains to be seen, as factors such as driving style and the use of regenerative braking have to be taken into account as well.
Considering that microplastics, particles < 5 mm in length, are now found practically everywhere on Earth, including in our own blood, it raises uncomfortable questions about how harmful they are exactly. This seems a valid question considering that one property of these plastics is that they take a very long time to degrade. If they hang around this long, then surely they do not interact with or harm ecosystems and the insides of our bodies?
As noted by
Although at this point in time there is no evidence to support the theory that micro- and nanoplastics are actively harmful to human health beyond PM2.5 pollution, indications are that at least in the case of tire wear, the additives that leach out of the fragments into the environment can do serious damage.
More Than Just Rubber
Tires are rather complex constructions. Rather than just natural, vulcanized rubber in a funny shape, their manufacturing involves the combination of natural and synthetic rubber, along with carbon black, silica and a range of antioxidants and antiozonants, the latter two serving to make the tire more resistant to UV and ozone exposure.
About half of a tire by weight is carbon black, which is a paracrystalline form of carbon. There is limited evidence (Group 2B) that exposure to carbon black may be carcinogenic, and at low levels it would seem to cause harm to the public’s health. The synthetic rubber in tires is usually styrene-butadiene rubber (SBR), which provides comparable properties to natural rubber when protected by additives, a common one being 6PPD (C18H24N2), which acts as both an antioxidant and antiozonant.
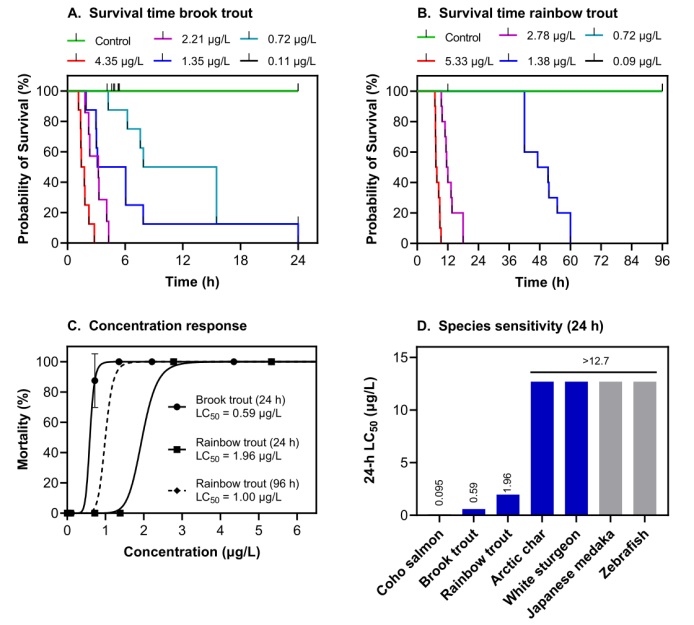
durations of 24 and 96 h, respectively. Median lethal concentrations at 24 and 96 h of exposure were interpolated for both species using (C) two-
parameter logistic regression and (D) compared with those of other previously studied species. All concentrations are based on measured
concentrations. (Brinkmann et al., 2022)
Recent research (Tian et al. (2020), Brinkmann et al. (2022)) has shown that it is this commonly used 6PPD additive which ends up being highly toxic to marine species like the coho salmon (Oncorhynchus kisutch) with an LC50 (median lethal concentration) of < 0.8 μg/L. The toxicity appears when the 6PPD molecule reacts with ozone to form a quinone form (6PPD-quinone). Multiple studies have now attempted to determine which marine species are the most sensitive to this 6PPD-quinone substance, with Brinkmann et al. finding that rainbow trout (LC50 0.59 μg/L) and brook trout (LC50 1 μg/L) are also very sensitive.
For these sensitive species, the displayed symptoms upon exposure to 6PPD-quinone in sufficient concentrations included increased ventilation, gasping, spiraling, and loss of equilibrium. Eventually the sensitive fish species in these studies would succumb and die. As noted by Brinkmann et al., 6PPD-quinone is found in significant concentrations in stormwater runoff and in surface waters along the US west coast at concentrations of ≤ 19 μg/L. Effectively this means that these waters are lethal to at least a number of species that are of ecological, economical and cultural importance in this area.
Fixing The Mess
Even as the ecological, environmental and health implications of microplastics are still hotly debated today, it is hard to argue with the well-documented health impact of PM2.5 particulates, not to mention that of 6PPD-quinone on marine species. The human health impact of tire wear will be most visible in the occurrence and severity of COPD and similar conditions near roadways and similar areas where significant amounts of tire wear occurs.
For marine species the health implications seem significantly bleaker, however. While some fish species seem remarkably unimpressed by even full saturation levels of 6PPD-quinone, for many others even fairly low concentrations appear to be invariably lethal. Since many of these sensitive species are of commercial interest, there is a real risk that the increasing presence of this molecule in marine environments may drive them into extinction, or at least make their commercial exploitation impossible.
Unfortunately, as noted by the U.S. Tire Manufacturers Association (USTMA), there is no known alternative to 6PPD that can easily replace this tire additive. In the meantime, mitigation methods are being examined, and motorists are being urged to maintain proper tire pressure to reduce tire wear. Hopefully before long we will find a replacement for 6PPD that addresses the toxicity issue.
As to the microplastics issue, this is an issue that sadly stretches far beyond tires. Even so, there are aspiring attempts to capture the particulates from tire wear at the source, using an electrostatic capturing method or air filtration attached directly near the tire. If successful, this might even help mitigate the 6PPD problem, though obviously not all tire wear particles are so easily captured, as demonstrated by the tire marks on every stretch of tarmac.
















Even if you can’t buy a smaller lighter vehicle you can drive in a more relaxed manner when appropriate to minimize rubber loss and get your car an alignment.
You will save money because your tires will last longer.
It is pretty bad.
That’s why I bought an atom.
Just one? I have zillions of atoms, probably more than I really need.
https://www.youtube.com/watch?v=WaWoo82zNUA
650kg/350hp
You should have bought a Proton, they are smaller.
A motorbike has ground-contact area of about two postage stamps, leans to steer instead of rotating half the tires, has half the tires to begin with, and doesn’t have as much rpm differential between them :) And my carbureted engine from the 70s gets 60mpg
My 2016 Chevy Volt got a special edition tires, which are thinner and lighter than usual. Manuf. claimed 40k miles lifespan. Nevertheless they served ~100k miles because of dynamic braking with energy recuperation back to battery. Combining ~40-45 miles pure electric range and 45mpg on gas – it’s much better than all my previous cars. The statement that tire wearing on hybrids and EVs should be worse than ICE cars – is wrong.
Other than some minor electronics issues, the Chevy Volt is a wonderful car. Shame they canned it.
What’s the shelf life though? It’s dangerous to drive on old tires that have gone all hard.
45mpg? That’s terrible! My petrol 2016 Peugeot 108 gets 75mpg if I really try (and about 65 if I really don’t). Cars should be smaller
that’s 45 MPG *without factoring in the electric drivetrain* – that is, if you ripped out the electrics and replaced them with the same mass of say, sand, the car would get 45 mpg.
75mpg on the Peugeot is *total drivetrain*
My car gets 26mpg kekw
My Ecopia tires on my EV wore out faster than any tire I’ve ever owned. I could hardly believe it. Switched to some basic old fart gentle ride less efficient rubber and I’ve gone 50,000 and still have 75% of my tread left and the range penalty was minimal.
Yes, but what mpg do you get in 12 inches of snow when the temperature is -5F ?
The same? Perhaps some energy would be lost to the temperature, which is known to happen to lithium-based batteries, but the Volt has an onboard petrol generator meant to compensate for its smaller battery (ironically, giving it a useful counter to the -5°f temperature.)
An on board petrol generator?
Our fossil fuel crisis is solved!
B^)
As I’m going to say 95% of vehicles sold globally can’t deal with 6″ of snow let alone a foot its hardly a relevant comparison.
Though I have seen the dirt bikers ride on loose and hard snows – quite spectacular to watch, and in skilled hands perfectly useable if very impractical for the daily commute…
As for the temperature in terms of a functional vehicle doesn’t make much odds, and in terms of operator comfort cold is much easier for a biker to deal with hot – can always add more layers, heated vests etc. But its usually inviting a Darwin award levels of foolish to ride without the safety gear.
Define “deal with” – you can certainly drive in 6″ as long as you have the ground clearance. You’re liable to get stuck though.
In 12 inches of snow, you’re not going anywhere in anything but a snow plow.
Really. Funny, I live in Montana, and have driven in over a foot of snow with the proper tires, and ground clearance. I’ve seen it so bad the only way to find your lane is with the bumps on the edge of the lane, and reflector poles. It gets bad enough that on the passes they actually put two reflector posts, one on top of the other, so you can see the edge.
After watching channels like just rolled in, I can say… you are asking a lot to people that can’t even keep the basic functionality of a car intact. Let alone something that “superficial” like an alignment.
The amount of literally wheeled dead traps in circulation it’s just frightening.
Can’t we add some sort of plastic or textile coating to car tyres so they don’t pollute? I wish more people aperciated bicycles because they are good for health and easy to recycle. I do 18 km trip to work every day, even when it’s -10C outside and so far I’m 38 and feel fine.
Sure would be nice if our cities were planned from the ground up with walking and biking in mind. I have to get in my car to go less than 1/16 of a mile because there is a 5 lane road where people blow the speed limit and there is an accident literally at least once a week, and those are only the ones that I know about. If i did walk or bike, I would have to go well out of my way to get to a cross walk and it is such a busy intersection it is not safe to cross in any way because people are concieted and could care less to even consider someone walking. Most of the time they fly up to the intersection and roll right over the cross walk.
It is both frustrating and asnine that I have to use my car just to cross a street safely.
The next closest crosswalk is nearly an extra mile out of the way, the next ine after that is at least 2 miles out of the way.
With the lack of consideration and the amount of accidents there is no possible way i could commute in anything small or eco friendly, let alone a bike.
Not to mention, only one state in the entire united states has made “jaywalking” legal, which most municipalities define as crossing anywhere but a crosswalk rather than the original definition of crossing from corner to corner.
All the good will and tech advancements in the world wont make one spec of difference until our legislators and legal system stop being so dumb and backwards.
Sounds like you live somewhere without proper snow in the winter.
Chicagoin here. Believe it or not, bicycles are more than capable of weathering “proper snow” winters. I myself was doing bike deliveries when average snowfalls here were 21″.
It’s highly dependent on whether the city bothers to clear and scrape the paths whenever it snows. Proper winter is when the snow stays on the ground for months, and gets packed into ice when you drive through it, so cycling becomes hopeless slogging and quite dangerous. On an unmaintained path, whenever there’s new snowfall it covers the raggedness of the ice below and you get hidden ice grooves that will swipe you out.
Northern Norway is easily traversible with a bike winter time, just get the right tires (studded). I commute about 10km every day, takes me about 30mins on me enike. It’s probably also a lot worse than most other places since we get a lot of percipitation, switches between freezing and rain (coastal(, high winds and plenty of hills.
HaD recently had an article about a textile on a tire.
IIRC, the textile was twine.
B^)
The measures that have been put in place to reduce the most harmful automobile exhaust emissions are there to reduce emissions of gases, which are their own problem separate from particulates.
There are two problems with microplastics: Particulates that get in the air, and ones that are flushed into waterways. Diversion of storm water from highways into groundwater recharge would provide these materials more time to harmlessly degrade. The problem with carbon black is that you’re breathing it. Where plants can metabolize it, it’s not undesirable. The larger molecules of additives would also have time to break down.
There is no evidence to support your assertion that these materials “harmlessly degrade”. In fact the degradation could cause more volatile and harmful byproducts to be produced.
And of course it really is beyond reason to expect that highway drainage will be modified as you say, the cost would be ridiculous.
Drainage has to be rebuilt periodically anyways because the culverts and ditches erode and silt over, and the materials are biologically degraded – they are metabolized into water and CO2 in the end.
Unfortunately this includes bicycle tires. Though the per-user amount is rather small compared to driving a car on the same duty.
Oh what about belts and hoses? Shoes oh my!
I like how absolutely no consideration is being given here to proper public transit infrastructure…
/s
“Not Just Bikes” is a YT channel that is essentially a free-at-home course on how to do such things right, replete with no-holds-barred examples and explanations of how many places get it wrong and why. The channel owner has a bit of very blatant bias towards Amsterdam in particular, but he not only makes no effort to hide it, he actually has, arguably, some pretty good reasons — the city implements both public *and* private transit far better than most cities even in continental Europe.
It’s actually not a bad idea, it’s just that here in America we’ve been doing it horribly wrong for so so long, I daresay we’ve forgot how to do it even *remotely* right, to the point that — as Not Just Bikes points out, repeatedly — we actually have laws (especially on zoning and on parking allocation) outright *preventing* a lot of “getting it right” in most places.
Bugger! This was NOT meant to be a reply to another comment. Would someone kindly fetch a wrench and fix it?
Also: obligatory ‘Edit’ button request to be ignored by staff as per usual. Not that it would help here!
I love the NJB YT channel. Recently visited Amsterdam, the place has fantastic transportation.
Lint from washing machines is actually a large contributor to the microplastics problem
It probably doesn’t help, but I keep a nylon stocking over the drain hose of our washing machine.
Every month or so, I dump the lint that has accumulated into the “waste to energy” trash.
Is this anti-drifting propaganda :-) For many decades some people have wondered why the roads do not have wandering dunes of rubber and brake pad dust.
There will never be an end to this. No matter what you do, there will always be a top polluter.
Great, now my tires are trying to kill me. It’s looking more and more like I won’t get out of this life alive!
(Chuckle)
Are you tired of life?
Is there a solution for being tired of inflation without reinventing the wheel though.
[Bigger chuckle]
Here in Ontario Canada we have a tier TAX. And then there is the Electronic tax.
For the electronic tax I spent $4 and paid $1.40 in TAX. I know that is for another story.
Back to this tier TAX here in Canada.
I’m sorry but I don’t know how much it is now.
I still don’t know how this tax is helping with this problem with the environmental problem we are haveing.
I do hope that there is a solution to 6PPD.
Every one be safe..
So, it’s tier tire tax.
Or a tire tier tax?
>one property of these plastics is that they take a very long time to degrade
One property of microplastics is that the smaller they are, the faster they degrade biologically. Soft plastics such as rubbers don’t really last that long before they’re degraded by bacteria – months to couple years depending on oxygen availability, temperature etc.
Solid long chain polymers are hard to digest by enzymatic action because the enzymes pick the polymer apart by the ends. When you have a solid macroscopic piece of plastic millimeters in size, it’s like a big knot where the ends of the polymer are embedded inside the mass with few to attack on the surface, so it takes a long time to break apart. But, once broken into smaller pieces that are actually microscopic, the ends of the polymers become available and the digestion by bacteria picks up considerably. As long as it isn’t toxic, they eat it like they eat naturally occurring polymers such as latex and lignin.
Example:
https://www.researchgate.net/publication/226907986_Microbial_degradation_of_tyre_rubber_particles
0.8 mm particles of tire rubber were reduced by 57% in 8 weeks by specialized bacteria in a stirring tank.
Looking up different articles online, naturally occurring bacteria and fungi degrade tire rubber somewhat slower with 10-20% mass loss within a year in mixed soil conditions. The process requires oxygen, so tilling the particles into the soil slows things down. Most of the research seems to be about what happens to larger chunks of tire rubber in a dump, so it’s sort of a worst case deal and not directly relevant to what happens with tire dust around roads.
So, we don’t have to worry about the microplastics at all? We just have to convince all of the particulate to find it’s way into a stir tank with some specialized bacteria?
Sounds like the problem is solved . . .
We have to worry that they should not be toxic to the environment as such. Otherwise they’re not that much different than falling leaves in autumn: polymers (lignin + cellulose) break down into microscopic particles that blow around in the wind and wash down the waterways, get consumed by organisms and vanish over time.
The task is to make tire rubber more like falling leaves.
Mind, hundreds of millions of years ago, trees developed lignin (a kind of plastic) that other organisms couldn’t readily digest, for one point that they couldn’t eat the trees. Mayhem ensued – trees were buried without decomposing and oxygen started increasing – until the bacteria and fungi evolved to eat lignin.
Already we have organisms that will eat almost all the plastics we’ve developed. Maybe we can’t wait millions of years for them to proliferate, so we should use plastics that are more edible by more organisms.
Then there’s also the simple fact that smaller particles have proportionally more surface area, so there’s more surface per mass to attach to.
One thing to remember is that if the tire doesn’t degrade, it’s going to work a lot less well: that friction is needed for starting and stopping.
i think your point is right but i wonder if it’s a real inevitability or just where we’re at today. i think for traction, what’s important is that it is pliable enough to conform to a surface decently well. i’m not sure if it’s the surface area that’s important or the fact that in order to move it would have to conform to a new surface. i do know that it’s going to inevitably produce some heat. but i don’t know, is abrasian necessarily inevitable? i kind of think it isn’t.
but it’d take some crazy material to be pliant like rubber yet not abrade as much as it. given that even brake pads aren’t much further along on abrasion (i replace brake pads and tires about as frequently on my bike, at least), it seems like it’s a long way off.
During the latter 1970s, Japanese cars had tires that could last 100k miles.
But they were lousy on wet or icy roads.
IIRC the biggest problem with 100k tires is they flat spot whenever the car is parked long enough for the tires to get cold. Then you bump down the road uncomfortably until they warm up. They never shipped.
I’ve owned a Honda 600N. Great fun. You could drive like Schumacher and not break the speed limit. You better know how to downshift. Bit of a deathtrap.
It’s much more fun to drive a slow car fast than a fast car slow.
The rear wheel drive Skodas from the communist era also had eternal tires, Barum, Drove 7 years with the tires and they never wore down. As with the japanese tires, these were bloody awful on wet surfaces.
Tires have three sources of friction: static friction, chemical adhesion, and mechanical grip. The first two involve molecular binding that always ends up transferring some material from one surface to the other, and the third ends up shearing material off the surfaces by abrasion.
A tire that has only static friction and mechanical grip is actually dangerous, because it has a sharp transition from rolling to sliding friction where both effects become greatly diminished. Adhesion effects that literally glue the tire to the road work under sliding conditions – that’s when you’re leaving skid marks on the road. Greater surface area helps with adhesion, which is why formula 1 tires are wide and made of a material that turns gummy when hot – so the car doesn’t just instantly spin out like the old cigar-shaped cars with skinny motorcycle tires.
rubber is weird, maximum acceleration, braking and turning is with rough 10% slip
> Solid long chain polymers are hard to digest by enzymatic action because the enzymes pick the polymer apart by the ends.
Not exactly true.
Enzymes can attach to a long chain anywhere they find a matching “surface” (enzyme specificity). See transposase, an enzyme that “cuts” and “pastes” directly in the middle of a DNA chain.
The problem of polymers is that enzymes need some electrostatic force to “attach” to the molecule. Most polymers are polar neutral, they don’t have enough (or any) polar groups like -NH, -COOH, phosphate, so they don’t ionize in water and there is no place for an enzyme to attach, not even at the ends.
As I understood, enzymes can more easily attach to points where the polymer branches, but not so easily to points where the strand is continuous. That’s also why some polymers are harder to break than others.
It also depends on how the polymer branches. But for this conversation it’s largely a matter of something called steric hindrance, which is other stuff getting in the way. Enzymes are usually massive compared to their substrates, so it’s like trying to get a little bead nestled up to a basketball so the basketball can do its thing, and if there’s lots of junk all around, like the bead is in a knotted up necklace, there isn’t enough force for the substrate to nestle down into the enzyme and get modified. The enzyme/substrate binding is driven by several forces, but none are particularly strong or at all long-distance, so save for the last couple of nm it’s largely driven by diffusion speed in the solvent and simple physical availability of the site.
But more generally, typically enzymes will target a molecular subunit of a polymer, and cut it off where it joins to the next one. That’s how enzymes/proteins are made, by linking together subunits into polymers, and similarly how things like starch are degraded into individual sugar subunits. So, it’s not branching per se, it’s just getting to the point where the subunit joint is.
Cellulose was so successful because it used a double branching off a single subunit that there weren’t any available enyzmes to break down, so when plants initially started using cellulose for cell walls they were invulnerable, and it took a very long time for bacteria to come up with an enzyme that had a way to attack that branching.
Ah, so it was cellulose instead of lignin where that happened. I got them mixed around.
hmmmm what if we invented a form of transportation using metal tires
i know metal won’t have a lot of traction on pavement but maybe we can keep them going where they need to go by making the tire fit into a kind of guide or rail, you might even be able to make more efficient and less polluting engines by building one big engine that pulls like 3 or 4 cars hooked together
the metal tire cars might not go exactly where you want to go, but once it gets you close, you can take rubber tire cars the last few miles
idk maybe that’s crazy
Sure it’s crazy… but it might just be crazy enough to work!
C’mon, kiddo, we’ve got a train to catch!
[music swell, up and under]
We already have this, it’s called the railroad. And those steel wheels wear too.
“But all the towns and people seem
To fade into a bad dream
And the steel rail still ain’t heard the news
The conductor sings his songs again
The passengers will please refrain
This train got the disappearing railroad blues”
-Arlo Guthrie
“Passengers will please refrain from using the toilet while the train is in the station. Thank you”.
Dvorak’s Humoresque.
So much for refraining the rest of the way. Bombs away.
Because until recently, the toilets dumped their contents onto the rails when they flushed.
Look up bro, i think this one went over your head
How about we build steel rails on major highways and have the cars also have a set of steel wheels for that? Then we can also use the rails to supply electricity to the cars and not have to oversize the battery to be able to make the long trips.
If tires will not produce particles they will not wear. If they will not wear, nobody will change tires periodically. If nobody will change tires periodically, tire manufacturers will lose a lot of money.
There are many simple solutions to reduce tire wear, like adding sand to tire tread (Cooper CS4 Touring, f.e., lasts much longer than other models without sand in tread, so wear less and obviously emit less rubber particles). But that is not what that all new hype about. You will buy worse tires, made from expensive “biodegradeable” “nature friendly” rubber, for much higher price more frequently and pay “microplastic tax”. Much more profitable scheme, than just adding sand to tread, isn’t it?
People are new oil. Deal with it.
It’s not a conspiracy – harder tyres grip worse.
If race teams could get hold of a tyre that gripped as well as the incredibly soft rubber they use but lasted a full race they would beat down the door of ANY manufacturer who thought they could make one and throw mountains of cash at them without question.
I’ve had cheap sets of tyres that were made from hard rubber, sure they last ages but it’s worse for the environment when your car flies off the road into a ditch at every corner. Good quality tyres have a ton of clever stuff in them that makes them grip well and last for a long time, sure you pay more per tyre but you get better value (and safety) in return.
> It’s not a conspiracy
Conspiacies exists. Especially among large corporations making everyday things. Check “Phoebus cartel” when incandenced bulbs manufacturers conspired to make bulbs that will last limited time for profit.
> harder tyres grip worse.
It is not about “harder tires” at all. It is about what exactly grip. Tiny sharp pieces of SiO2 grip not worse than rubber, additionally they could grip even on snow/ice. Rubber where that particles reside could be as soft as you like. I mentioned exact model of tires, you could easily check it yourself. You could see that sand particles in tread using magnifying glass or microscope. They are not harder, they are even softer than many others, but have much lower treadwear because of different gripping substance. Unfortunately Cooper dropped that tech “Siica something”, I don’t remember, so you will have to search that tires at shops, may be some still have some in stock.
> If race teams
It is not about race teams. It is about usual tires for usual cars. Some tires wear twice (I would tell 3x, based on my experience) less than others with absolutely same common tire characteristics. The only difference is a sand in a tread. They are not harder, they are not slower, they are not even more expencive.
If you make a tire with tread fully covered by tungsten carbide studs (like on winter tires), you will get a tire that will be soft, but will not wear. Yes, it will be extremely loud, but it is OK for the proof of concept. Then, make that studs much smaller along with growing its number. Replace studs with some hard particles like quartz or corundum. Finally, you will get a tire that have same grip, same flexibility, but nearly no wear because it is hard particles contacting with the road (rocks and sand in asphalt) and not a rubber.
There is another way to noticeably lower treadwear – use rubber particles (say, from used tires) in asphalt instead of sand and rocks. But it is a very expensive and long way to reach the goal of lowering tire wear.
You see? Either you make tread harder relative to hardenss of road, either make the road softer. Today, you just rub rubber against sand and rocks. Obviously rubber wears a lot.
Whenever two parts are rubbing, you design one to be hard and one to wear. Simplified but basically true.
Your suggesting of making the road the wear surface isn’t well thought through. Think about it. Those already exist, they’re called dirt roads.
Particles of silica have no chemical adhesion to the road surface, so they have the same effect as hard rubber: great when you’re just rolling along, but dangerous when the tire starts to slip. Part of what makes a good tire is being a bit tacky.
Also, what you’re effectively proposing is making the surface of the tire like sandpaper, which will wear the road surface faster – there’s a reason why studded tires aren’t allowed in the summer time. That would simply switch from one problem to the other – roads are expensive to replace, and the bitumen dust is equally an issue.
> That would simply switch from one problem to the other – roads are expensive to replace, and the bitumen dust is equally an issue.
Oh, no, I purposed prohibited thing – to make things cheaper for the people! Exactly. See, how you subconsciously care about miniscule benefits of government/state/corporations at the expense of the people.
Really, roads have to be repaired often in any case. There are many other reasons for that, from the weather conditions to the depression of asphalt due to the weight of cars and trucks. Rubbing wear is insignificant in comparison to all that things. Nothern countries use winter tires with studs most of the time and there are no such reason for road repairs like “weared by studs”.
Why are you so care about government/state/corporations? When their problems and concerns become yours?
Stanson: Sometimes you’re Dunning, sometimes your Kruger.
Today isn’t a good day for you. Don’t try to defend blithering stupid. ‘The people’ pay for everything, government produces nothing.
>Nothern countries use winter tires with studs most of the time
We get ticketed if found driving studded winter tires when there’s no ice on the roads. There’s about a month of transition time between the seasons. They’re explicitly banned in the summer time because they turn roads into sand.
“People are new oil.”
“It’s made of people!”
-Soylent Green
Sand dust is not good for the lungs either.
But our lungs are made of star dust!
B^)
and our wishes are made of angel dust
no, wait, that’s not right
> Sand dust is not good for the lungs either.
But there is much less sand dust wear out than rubber dust. So, overall, sand dust looks better. Also, do you know that sand dust is an approved E551 food additive? You exposed to much higher sand dust concentration than you possible could be exposed during years of tire wearing just by opening your can of Pringles (or other “potato” chips). And then you literally eat all that sand dust, breathing it in in process. And you really worry about some potential sand dust from SiO2 enforced tires?
From the other side, you traded our flying cars to 140 characters (or whatever many now) in twitter. So, whatever.
Eating a peck of dirt and inhaling a cloud of dust are two different propositions.
s/capitalist/globalist
Another reason not to own a car and take a bike instead! Or rent a car! You will own nothing and you will be happy.
B^)
Nah, I’d rather own my car and drive it around for fun.
This is clearly one of the gravest threats we face. This rubber tire story ties in nicely with reports the World Economic Forum calls to reduce private vehicles by eliminating ownership. I fully support forced confiscation of private vehicles except of course for the famous and elite because of their superiority and importance. The hoi polloi proletarians should be at home or work in their worker uniforms. Let them ride bikes or take a bus.
I suspect that a large concentration of self driving cars on the road will easily cut that number in half. Having cars communicate with each other, and change speeds to be able to go through intersections as a group without having to brake hard or accelerate hard will allow owners to use tires with harder compounds that will at least double the lifespan of tires on these vehicles (imho).
Yes this is right.
And one way or another, driving will move to a pay-per-use model, rather than as it is now, a large capital investment and then unlimited use (apart from just gas costs).
When you pay per trip, people will make sure the trips are worthwhile – no more driving around just to see if your friends are home, or just for fun.
Driving around is indicator of a freedom. You can go everywhere anytime. When this falls, you will become oficially a bondman, or how they were caled in medieval times. First tires ban, then other technologies until full dark age again. It will just roll back few centuries of development, but i guess no one cares anymore.
Restrictions on private mobility cause problems of labor mobility, which leads to economic issues and higher cost of land around transportation hubs. Central planners like the idea because they can just dictate where everything goes without worrying about the cost – a free market cannot operate in a situation where people are unable to move freely.
Most people today won’t notice or care until it’s too late.
The powers in control want you to watch more utube, drink beer, and not think about it.
How will self driving Cars (when communicating with each other) transmit the middle finger?
Why not use actual rubber? After all, it’s carbon neutral, just add some sulphur.
Secondly, at least with the petrol engines eventually eliminated, catalyst particulates will die away (I’m mainly talking about PM2.5 and smaller bits of Platinum). And along with it, the current epidemic of asthma will eventually disappear (you do know, that platinum miners get asthma as an occupational disease?)
Exhaust particles are more an issue with diesel engines, which will not go away for a long long time. No way you haul containers any long distances with an electric vehicle.
Wired electric will handle long distances easily.
I always wondered how toxic the smoke from a burnout at the end of each Nascar race was. Not to mention the rubber that builds up on the track changing the groove throughout the race. Maybe Nascar could study the problem and come up with solutions to recover the spent particles.
Maybe street sweepers could capture these particles on busy roads.
What about all the spectators inhaling the tire particles?
They are just nascar fans, nobody cares. Lols.
There’s a guy that recovers platinum and palladium from street sweepings…
When I was younger my family was very poor. Oftentimes we would not have electricity or running water. My dad always had some kind of beater car to get to work with. My siblings were no better off and also had beater cars. I say this because the cars litteraly had 3 kinds of tires (tyres for those across the pond). 1) drivable tires : these were tires with tread on them. 2) baloney skins : these were tires with no tread on them (think racing slicks not in a good way) 3) may pops : these were tires that the wires (radial) or strings (bias ply) were sticking out and will leave you stranded with no notice. Every car had a spare tire ,but, they were the worst of the may pops. My dad was driving on Roosevelt rd. Melrose Park Il.USA when he hit a large piece of brass in the roadway. It punctured the passenger side rear across the tread with a fist sized hole. Dad was so angry that a drivable tire was destroyed and had to be replaced with the spare may pop. I like to think that we got every possible mile out of our tires before sending them to tire heaven.
P.S. I say this not to make you feel for me but rather to show that a tire wear strip is just that. It means you have 10-20,000 left on them.
P.P.S If your car is rear wheel drive once you get to baloney skin state watch out for hydroplaning.
Baloneys aquaplane on a front wheel drive also. Granted, it wont fishtail, but losing steering in a bend is disconcerting.
Let’s check some math. I have an EV, a Renault Zoe 2016 (bought in early 2017). Tyre specs: Width=205mm, Profile=0.45*205mm = 92.25, Rim=17″=43.18 diameter. Thus the surface area of the tyre is: (9.225*2+43.18)*π*20.5 ≈ 3969cm². My guess is that the tread grooves are about 25% of the surface, which means that the effective surface area is about 3000cm².
A new tyre has a tread depth of 0.8cm and was replaced at 0.3cm, so 0.5cm was lost , giving a volume loss of 1488cm³ over 78,000km, or about 0.019cm³ = 19mm³ per km. In fact we have 4 tyres, so this is really about 80mm³/km.
Or we can compare this against the figures above. Car tyre rubber is 390 kg/m³ = 0.39g/cm³. We lost 1488cm³*4 over 5.5 years = 1190cm³ per year = 464g per year.
Now let’s look at the article’s claims:
“Their estimate comes down to between 0.23 to 4.7 kg/year, with a global average of 0.81 kg/year/capita.”
So, our car, an EV, is at the low end, at just over half the average.
“An awkwardly situated elephant in the room that further complicates this matter is that the weight of BEVs tend to be higher than that of ICE cars due to the weight of the battery pack.”
But the elephant in the room isn’t the tyres, but CO2 emissions.
Our car generates 464g of particulate pollution per year, but a typical UK combustion car generates 138g CO/km and we drive about 7400*1.608 = 11899km/year => 1.64 Tonnes of CO2, or about 3538x the weight.
That’s the elephant in the room.
A Renault Zoe is a very small car (supermini), weighing in at just 1,468 kg. Yet, an equivalent petrol vehicle, such as the Renault Clio, weighs in at 980–1,071 kg. You’re hauling an extra 500 kg of batteries and related materials along, which has a great impact on tire wear.
Also, CO2 is not particulate matter, it’s a gas. Not a relevant comparison.
“there is no known alternative” OMFG. Where have we heard this before? Maybe, approximately everywhere? How about tetraethyl lead? Rare earth for magnets? Lithium for batteries, tantalum for capacitors? CFCs? Halons? Lead in electronics? There are TONS of options. They just hit the bottom line of companies oh so slightly, and may reduce performance slightly, that’s all. So you have to do things a little differently like replace your tires a little more often. That’s basically it. There are *definitely* options. Finding them won’t be trivial, but there have always been there, every single time we have heard this BS.
Although thoughtful, particulates from tire wear is not at the forefront of our most pressing issues.
How about doing some actual research into:
1. Where do the raw materials for batteries come from?
2. Are those materials in countries that would oppose the free speech happening on this site right now?
3. When those materials are made into batteries, what it the environmental cost?
4. When those batteries reach end-of-life, where do they go, are they recycled?
5. Where are they recycled, and what is the environmental cost?
The success of EV technology is critical to just about every living thing on this planet – IF WE DON’T SCREW IT UP.
Learn about the process and pay attention to where the greedy will cut corners.
Don’t just buy into the dream that the advertisers will sell you. Demand answers to the questions that make them uncomfortable.
Educate yourselves and understand that this technology will carry humanity forward, but the amount of collateral damage is still up to us.