Hydrogen fuel is promising, and while there’s plenty of hydrogen in the air and water, the problem is extracting it. Researchers have developed a way to use aluminum nanoparticles to rip hydrogen out of water with no additional energy input. It does, however, require gallium to enable the reaction. The reaction isn’t unknown (see the video below), but the new research has some interesting twists.
Aluminum, of course, is cheap and plentiful. Gallium, not so much, but the process allows recovery and reuse of the gallium, so that makes it more cost-effective. There is a patent pending for the process and — of course — the real trick is making the aluminum nanoparticles. But if you have that, this is a simple way to extract hydrogen from water with no extra energy and at room temperature. Since the reaction of creating aluminum oxide and releasing hydrogen with gallium is pretty well-known, it appears the real research here is determining the optimal properties of the aluminum and the ratio of aluminum to gallium.
While gallium isn’t a common item around the typical hacker’s workshop — unless you count the stuff bound up in semiconductors — it isn’t that expensive and it is relatively easy to handle. Hydrogen, though, not so much — so if you do decide to use this method to produce hydrogen, be careful!
We’ve seen gallium robots and even an antenna. So if you do get some of the liquid metal, there are plenty of experiments to try.

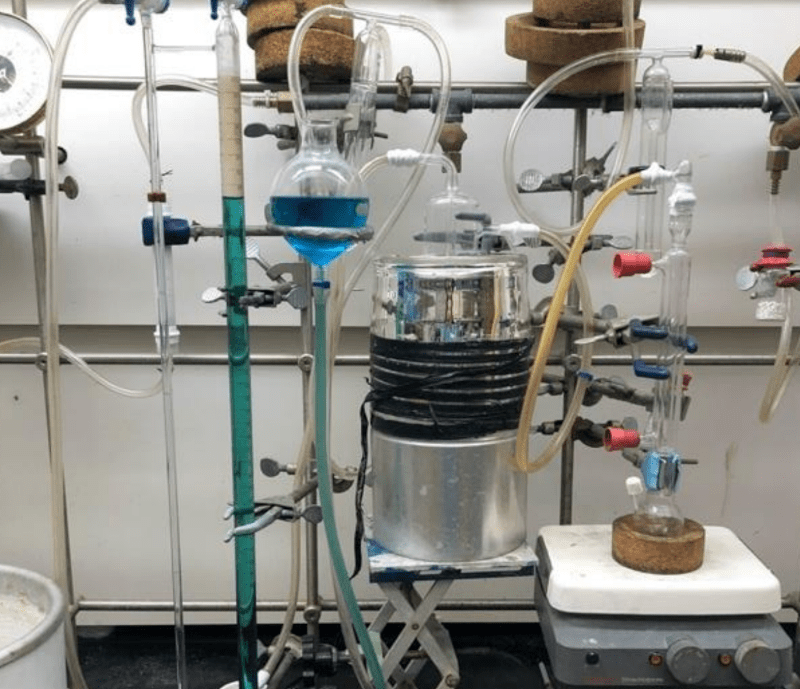














“There is a patent pending for the process and — of course — the real trick is making the aluminum nanoparticles. But if you have that, this is a simple way to”
No, the process shown here *creates* the aluminum nanoparticles. They literally used off-the-shelf aluminum foil. From the supermarket.
How energy intensive is converting the aluminium oxide back to aluminium metal, what process is most efficient? If it is electrolysis and heating, wouldn’t it be more energy efficient just to split the water in the first place.
Don’t get me wrong, it sounds interesting – but I am just not clear on how well it would work as a system for generating fuel.
This. As you point out, reducing aluminum oxide into aluminum metal costs a lot of energy.
I reached the same conclusion than you.
Aluminium hydroxide.
Simple electrolysis is all you need to produce hydrogen from water… the efficiency lies in the conductor reluctance and design vs just simple controls on substrate composition…. anyhow why bother with this process where you have to go back and try to reprocess used or spent? Electrolysis is also close to instant on instant off reaction within a normalized safe design… no run away conditions or uncontrolled chemical processes… maybe use this to start hydrogen production to start gas collection then start generator running on collected to swap to Electrolysis long term on a closed biodome system to save on shipping big batteries to Mars…? Solar panels work too though.
They DON’T recover the aluminum, it is ‘consumed’ in the reaction. Probably accumulates as ‘ash’ in the bottom of the reactor. The source of the aluminium would be all the non-economic recyclables.
It all sounds terribly wasteful and inefficient. Which is at least on-brand for hydrogen as a transport fuel.
Why reduce the alumina? It can be used elsewhere. e.g. refractories.
The energy input happens when you refine the aluminum. From TFA linked through TFA, (https://news.ucsc.edu/2022/02/hydrogen-production.html) they’re getting 90% of potential hydrogen for the mass of aluminum. Aluminum refining isn’t terribly energy efficient, but I wasn’t able to quickly find an estimate of efficiency.
Where this has potential is as an energy store. You can possibly use this to “transport hydrogen” virtually by making it easy to generate from water on site.
IE: hydrogen refuelling station could be set up similar to a gas station, with deliveries of this composite, and a pipeline for water. If recovery of gallium is easy enough the deliveries could be only aluminum.
+1
Hydrogen must be compressed to a liquid be useful as a portable fuel at around 700 bar. This takes a lot of energy and is a dangerous process not likely to ever be found at your corner filling station. Liquified H2 is trucked to current H2 filling stations. Hydrogen is very small molecule and is difficult to contain so leaks and explosions are always a possibility.
I’m guessing that any such hydrogen generation stations would be a nasty eyesore, too large, too dangerous, much too complicated to be operated by minimum wage gas station operators or ever be allowed in your neighborhood.
It’s not clear how much raw aluminum-gallium raw material is consumed, and how must “slag” is created per gasoline gallon equivalent of compressed H2, but clearly some volume of those two materials will move in and out of each station. My chemistry is too rusty to do that calculation. I’m sure somebody here can do that math. It would be interesting to know …how large a reaction vessel, how large a compressed h2 tank, and how many pounds raw material used, how many pounds slag created would required to create and store 5,000 gasoline gallon equivalent of compressed h2
Can you avoid high pressure by just driving around with water and your metal alloy tanks under the car, just like current on-vehicle fuel tanks? Foot pedal connects to a water bubbler “throttle” into the bottom of the alloy tank, top of tank vents to engine intake manifold?
The problems start when you have an accident and the powder keg ruptures all over the road. It’s the same issue as with MOFs and other attempts at storing hydrogen in some solid form – they react with moisture in the atmosphere, or oxygen, and spontaneously set themselves on fire.
click through to the actual paper
The technique outlined here produces 130 mL (5.4 mmol) of hydrogen per gram of alloy.
Fuel cells do not require high pressure liquified hydrogen, thats just the easy way with yesterday tech to drag around a bunch of hydrogen. Commercial vehicular PEM operate at 4bar(58psi)
A typical hydrogen fuel cell produces 1.8 kWh/L of hydrogen. That means that a teslas 85kwh battery would require 47L of hydrogen from full to flat.
1 liter of water = 1 kg of water. Molar mass of water = 18g, of which H2 = 2g, O = 16g. So 2/18 = 1/9 = 11%. So one liter of water gives 110g of hydrogen gas, which is about 1.3 cubic meters of gas at normal temperature and pressure, compressed to the requisite 4bar, resulting in 22L of compressed hydrogen per liter of water.
So 2.14L of water and 362 grams of alloy…241.33 grams of gallium.120.66 grams of aluminum….seems to cover everything but the weight of a fuel cell capable of processing at a rate equal or exceeding draw.
Sorry if my numbers are off anywhere….someone please correct me if you see flaw in my maths.
It would be safer to try for a dry compound or (relatively) low pressure storage system for the gas. 500 gge storage is more practical at a city gas station, with the rest converted to electrical storage. ?
I have an electric car and a giant battery hybrid Volt from shabby. I cannot tell you how enjoyable it is to not go to gas stations and give money to some giant conglomerate. All these hydrogen pushing refuel extensions discussed me discuss to me. If it’s not airplanes or busses or the people unfortunately live in apartments I think all the suburban items in country folk we just love to never visit a gas station again so I say nay to hydrogen
DoE did look at various issues around using aluminium for hydrogen storage and had published a paper on it: https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/aluminum_water_hydrogen.pdf
When I saw this article, first question was “does it also produce oxygen?”, and they seemed to avoid addressing that aspect.
Because hey, if you just want to get the hydrogen out of the water, all you need is a supply of elemental sodium.
Getting hydrogen by turning aluminum into aluminum oxide is the wrong direction. Might as well take all the electricity used to obtain that aluminum directly into the water and electrolyse it the old fashioned way
But there are tons of aluminum that already exists so that energy is already spent.
Yeah .. huge mountains of unusable scrap aluminum are everywhere. Now we know how to get rid of them 🤣🤣🤣
It ‘s a nonsense to now throw away the aluminium oxide – to complete the cycle it has to be converted back to the metal element.
Aluminum is absurdly recyclable. One of the easiest metals to recycle, especially when compared to the energy input required to make new from aluminum oxide (which this process turns the aluminum into).
Spending the embodied energy in aluminum on energy transport may be worthwhile, but I think it comes down to a case by case basis.
But aluminum is a hell of a storage medium, in 100 years aluminum will still have 100% of the energy used to make it “stored” in it, how much energy will be left in even the best battery if it hasn’t been charged in a century?
LOL 🤣🤣🤣
Yes it creates oxygen as well, but this is theoretically grabbed by the aluminum to create the resultant aluminum oxide “waste product” (at least hopefully enough to prevent booms)
I recall BMW making a hydrogen car a couple of decades ago that used aluminium reacting with water to liberate hydrogen. The ‘cost’ of the input of course is creating pure aluminium in the first place, nanoparticle or otherwise.
So you want to put a bomb into your bomb to run your bomb of a car? There is no way this will take off once our overlords realise the danger they would be in from their serfs having access to the materials required to make thermobaric weapons.
@Al Williams said: “We’ve seen gallium robots and even an antenna. So if you do get some of the liquid metal, there are plenty of experiments to try.”
At 23.0 °C, 73.4 °F on the surface of Earth gallium is a solid. Gallium melts at 29.7646 °C, 85.5763 °F.[1]
1. Gallium
https://en.wikipedia.org/wiki/Gallium
There’s one big elephant in the room however, The reaction of Aluminium and water is -highly- exothermic. the bulk of the reaction energy being dissipated as heat.
Ironically, it’s actually more efficient to use aluminium to boil the water in it’s reaction vessel and create steam than it is to extract the Hydrogen from the reaction.
The amount of heat energy from the Aluminium and Water reaction is something like 3 times the resultant energy of the Hydrogen produced.
Both the exothermal and Hydrogen components of the reaction could be used to create usable power, but there additional challenges.
The reaction is quite happy to occur at high temperature, 400 degrees C or more, which suggests that the reaction has the potential to generate supercritical steam for a small turbine unit.
The greatest difficulty would be the management of superheated pressurised hydrogen gas in the reaction vessel. Being at such high temperature, the Hydrogen will quite enthusiastically ignite if there’s any free oxygen present. It may be appropriate to use this also in a multi stage gas turbine unit.
I wonder how feasible it is to capture the superheated steam and Hydrogen, flow the combined mixture though a steam turbine stage, condense the water out at the end of the steam stage as a closed loop and then tap the now much cooler hydrogen and pass that through a gas turbine stage.
Gallium, is a byproduct of aluminium production anyway so that’s not much of an issue.
I wonder if a gas turbine with steam as the working fluid is feasible? ie a steam turbine with a combustion chamber?
It would need to be “carburated” with air, of course, to allow the hydrogen to burn, but the result would be very superheated steam.
Water injection into jet engines used to be a thing (hence the brown exhausts of the 707 at takeoff)
I suspect that this would be a good way to create nitrous oxides, though, so not as clean as one might wish for.
I think you are right about suitability for a stationary or large vessel powerplant with CPH. The challenge is to get hydrogen adsorbed out- the aluminum?
It’s a non sense. You don’t keep energy without spent the same or more in advance.
Aluminum is abundant in nature but not in metallic form. To get metallic aluminum you must spend more energy than that you obtain from the hydrogen generated.
It may be useful only as energy storage not as energy font!
And there’s also the issue of the energy and equipment needed to produce large amounts of nanoaluminum from aluminum stock. It is neither easy nor cheap.
“Hydrogen fuel is promising”, the people who’ve been watching the recent SLS launch attempt already know better.
H2 is very difficult to keep from leaking. Even rocket scientists with multibillion dollar budgets struggle .
This whole idea is complete bullshit. Let me show you why.
1 The article in https://www.sciencealert.com/clean-fuel-breakthrough-turns-water-into-hydrogen-at-room-temperature starts with
“Hydrogen fuel promises to be a clean and abundant source of energy in the future – as long as scientists can figure out ways to produce it practically and cheaply, and without fossil fuels. A new study provides us with another promising step in that direction…”
This promise alone cannot be kept. Aluminum is produced by molten-salt electrolysis. The aluminum is generated at the cathode and oxygen at the carbon anodes. This burns the anodes to CO and CO2. The production of 1kg of aluminum therefore consumes about 0.5kg of coal – a fossil fuel.
2. The bigger problem is that the electrolysis mentioned above consumes electricity, almost three times as much as producing hydrogen by direct electrolysis of water! The math on this:
The reaction in which aluminum with water produces hydrogen:
2Al + 6H2O → Al2(OH)3 + 3 H2 (source: https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/aluminum_water_hydrogen.pdf).
On the right side of the equation are 3 moles of H2, with a mass of 6g. In order for there to be 1000g here, the equation must be expanded by 1000/6 = 167.7. On the left side, the amount of aluminum to be used is 2*167.7 mol, corresponding to 2*167.7 * 27g = 9kg. (Correspondingly, the amount of water needed is 18kg).
The electrolysis to produce 9kg of aluminum requires 9*15.7kWh = 141kWh. (Source: https://de.wikipedia.org/wiki/Aluminiumh%C3%BCtte)
The direct electrolysis of water needs only 53kWh (source: https://www.gasag.de/magazin/neudenken/wie-viel-strom-fuer-1kg-wasserstoff ).
141 kWh >> 53 kWh
3. It is argued that scrap aluminum could be used for this process. This is nonsense, because it is much more reasonable to recycle this aluminum directly, because then the energy-intensive electrolysis is not necessary (https://en.wikipedia.org/wiki/Aluminium_recycling ). And waste aluminum of such poor quality that it cannot be recycled is certainly also unsuitable for producing hydrogen in a gallium-aluminum reactor.
I.e. for hydrogen production this process is a waste of energy.
4. How does it look for an application as energy storage? According to https://web.archive.org/web/20120222122120/http://auto-presse.de/autonews.php?newsid=91335 , the pressure tank for the Opel HyrdoGen4 weighs 125kg with a capacity of 4.2kg H2.
To produce 1kg of hydrogen, 9kg of Al and (according to the sciencealert article three times the amount of gallium, i.e. 27kg) are required. In addition 18kg of water are needed, in total 54kg. To produce 4.2kg of hydrogen, raw materials of 227kg are required. The weight of the container needed here and of the equipment for the removal of the reaction heat is not even taken into account.
227+ kg > 129 kg
5 All these considerations do not take into account that aluminum production is anything but environmentally friendly: https://www.global2000.at/aluminium
Definitely hit all the points I could think of, not new and not environmentally friendly.
For old fashioned electrolysis, what are best electrodes made of? ( I’ve been experimenting with stainlessness steel.)
First, I believe the mysterious Greek Fire of ancient times was actually pure sodium. It is a metal, and the Greeks would have rendered it by using an archaic solar oven or parabolic reflector similar to Archimedes’ death ray to melt salt. When sodium is purified it combusts on contact with water and that is what the Greeks would have launched at enemy ships.
Now, if you take a 1 meter magnifying lens and use it to focus sunlight, a heat sink could reach over 1000 degrees almost instantly. If an adjacent chamber is equipped with tesla one-way valves to release the gases inside, it will become a hot vacuum when it cools slightly. Adding steam to that hot vacuum chamber will cause the water vapor to fracture into hydrogen and oxygen gas. If salt water were distilled from the chamber then it would result in a hot vacuum lined with molten sodium, which would ignite the hydrogen and oxygen from the steam when it was reintroduced. This process could fire a cannon, or if it were over-pressured could potentially become an atom bomb. It is possible to use solar energy to burn seawater as fuel!
A nuclear reactor typically burns no hotter than 700°F and is used to generate steam to spin turbines. With a lens 1.5 meters in diameter, a temperature of 2000°F is easily achieved. An array of lenses and tubes as big as a football field utilizing concentrated solar energy and seawater would be fierce competition for any nuclear plant 6-12 hours a day, without the radioactive toxic waste. Given the vast potential present in a simple salt brine, burning our limitless seawater as fuel with a meter-sized magnifying lens seems like a viable alternative source of energy.
We could convert the water to hydrogen and burn that, but an even simpler option would be to use a pool (or brick) of hot NaCl at around 1000°F as a heat sink for pressurized distillation of seawater. Simply drop some saltwater into the pool; the steam is distilled and the salt stays behind as part of the mechanism. Also, since salt can be used to store heat overnight the machine could remain operational 24/7 once established. Lens-powered solar stations could move ocean water inland via steam pressure through a pipeline; this would solve both the energy crisis and the water crisis at the same time.
Thanks for reading