If decades of cheesy sci-fi and pop culture have taught us anything, it’s that radiation is a universally bad thing that invariably causes the genetic mutations that gifted us with everything from Godzilla to Blinky the Three-Eyed Fish. There’s a kernel of truth there, of course. One only needs to look at pictures of what happened to Hiroshima survivors or the first responders at Chernobyl to see extreme examples of what radiation can do to living tissues.
But as is usually the case, a closer look at examples a little further away from the extremes can be instructive, and tell us a little more about how radiation, both ionizing and non-ionizing, can cause damage to biochemical structures and processes. Doing so reveals that, while DNA is certainly in the crosshairs for damage by radiation, it’s not the only target — proteins, carbohydrates, and even the lipids that form the membranes within cells are subject to radiation damage, both directly and indirectly. And the mechanisms underlying all of this end up revealing a lot about how life evolved, as well as being interesting in their own right.
A Radical Proposal
Strangely enough, the main target for ionizing radiation in the cell isn’t any of the usual suspects like DNA or protein, but something quite unexpected: water. It makes sense when you think about it; on average, 70% of each cell is made up of water molecules, so it’s by far the largest target in terms of volume. Water absorbs most of the energy transferred to cells by radiation, whether in the form of photons — gamma rays, X-rays, cosmic rays, and ultraviolet light — or particles — alpha rays and beta rays, speeding neutrons, etc. And the changes that this energy transfer induces in water molecules can be responsible for dramatic biological effects.
When a water molecule is struck by an ionizing event, it leaves behind a positively charged species and a free electron. Both of these are quite reactive, and set off a cascade of reactions that can result in the production of free radicals, which are basically molecules that have an unpaired electron. The primary free radical that results from the ionization of water is the hydroxyl radical, which is one hydrogen and the oxygen from the original water molecule, with an unpaired electron on the oxygen. Hydroxyl radicals and related products of ionizing events are known collectively as reactive oxygen species, or ROS.
Thanks to that unpaired electron, hydroxyl radicals are so reactive that they’re virtually guaranteed to react with something within the diameter of only two water molecules from the ionization event, a very small distance indeed. That’s pretty bad news, because what the hydroxyl really wants is to hook up with a proton so it can be plain old water again, and it doesn’t care where it gets that proton from. That can spell doom for something like DNA, which is mainly composed of the five-carbon sugar deoxyribose; when a hydroxyl radical pulls a proton off this sugar, it leaves a lesion on the backbone of the DNA double helix that makes it prone to breakage.
No matter what the target is, biological damage that results from radiation-induced oxidative stress is called indirect damage, since the energy of the original radiation is transferred through the intermediary of free radicals. It’s estimated that 70% to 80% of radiation damage is indirect damage, which again makes sense because of the amount of water in a cell.
Holes In Bones
Biological macromolecules can also incur direct damage from radiation, and depending on the target, the results can be catastrophic. This can result in much of the same kind of damage that oxidative stress reactions cause, except without the limitation imposed by the narrow window of opportunity that hydroxyl radicals have to act. What’s more, because of the way DNA is packed in cells — each cell in your body has over a meter of DNA; to pack it all in, it’s wound tightly around proteins called histones — it’s likely that an incident photon of ionizing radiation can cause more than one lesion on a small stretch of DNA. This is compounded by the actual structure of DNA — despite the simplified cartoons, DNA isn’t a ladder, but rather a double helix with opposite strands actually in very close proximity to each other — which makes it highly likely that direct radiation will result in a double-strand break in DNA. The information-containing bases inside the double helix are also subject to direct radiation damage.
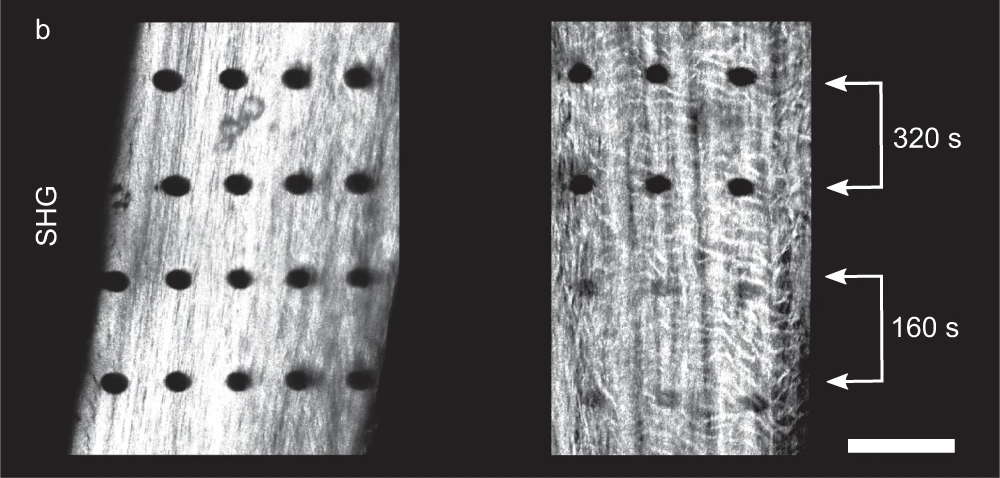
While DNA gets a lot of attention, it isn’t the only potential target for direct damage from radiation, nor is it necessarily the most important one. Proteins are also subject to damage, sometimes visibly so. Recent experiments have actually shown the physical track of high-energy X-rays as they passed through samples of bone, showing up as a series of tiny holes where the radiation destroyed collagen, a tough, fibrous protein found in structural tissues. The damage caused by the X-rays is thought to have been amplified to some degree by the mineral crystals of calcium and phosphorus in the bone, resulting in damage beyond the original path of the radiation. Although non-structural proteins, like enzymes, were not studied here, it can be assumed that they’d suffer the same kind of damage from direct radiation, with the same kind of amplification being possible.
Bind-ed By The Light
It’s not just ionizing radiation that causes direct damage to biological macromolecules. As anyone who has ever had a sunburn knows, ultraviolet light can cause quite a lot of damage too. While DNA is actually quite efficient at protecting itself from UV damage — most of the energy in UV is just converted to heat by DNA — some of the UV slips through to the information-coding bases inside the double helix. Here it can form what’s known as pyrimidine dimers, where adjacent pyrimidine bases — thymine (T) and cytosine (C) — become bonded together covalently. This happens when light in the UV-B range strikes the carbon-carbon double bonds in the ring structure of the pyrimidine bases. The result is that the two adjacent bases are joined together through a four-carbon ring, called a cyclobutane ring:
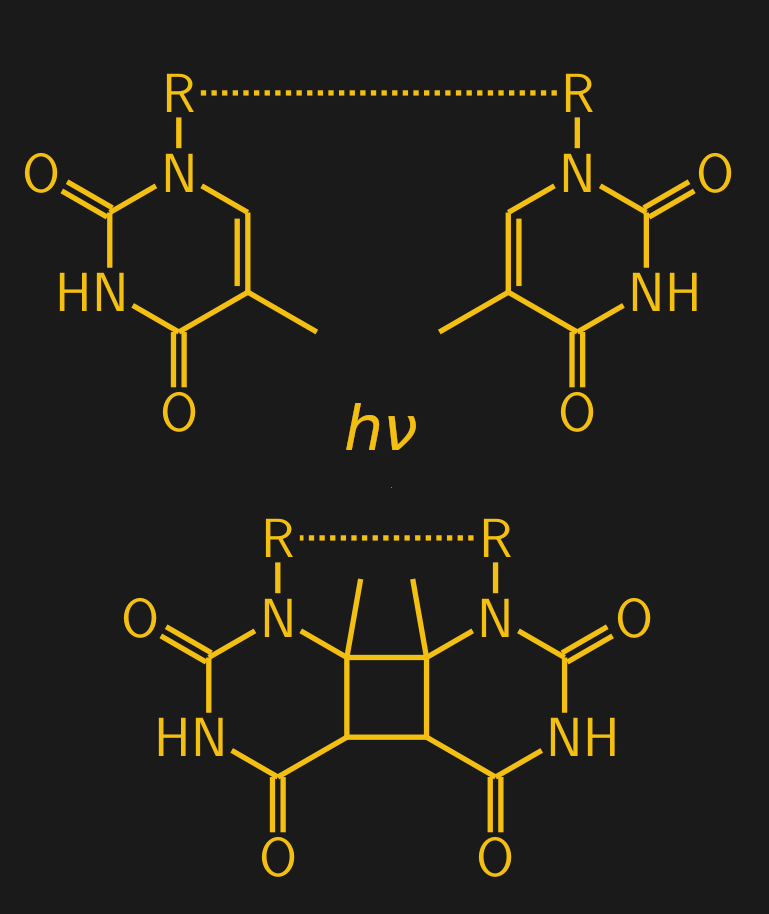
When a dimer forms, it introduces a conformational “kink” in the DNA backbone, designated by the “R — R” in the diagram. Normally, thymine (T) on one strand of the DNA double helix binds with adenine (A) on the other strand, but the formation of a dimer leaves those A residues unmatched. The whole thing is a messy situation that presents a number of challenges to the cell.
First is the problem of DNA replication. Normally, an enzyme called DNA polymerase rides along the length of a DNA strand, unzips it, and makes an exact copy of both strands. The kink induced by a thymine dimer makes it hard for DNA polymerase to move down the strand, potentially slowing down replication or even stopping completely at the lesion. Luckily there are variants of DNA polymerase that have evolved to deal with thymine dimers; unfortunately, they tend to be a bit error-prone, stuffing any old base in the growing DNA strand rather that the pair of adenines it should. This results in changes to the genetic code in the new strands of DNA, which can be a very bad thing indeed.
There’s also a problem with transcription, which creates the messenger RNA (mRNA) template that’s used to direct protein synthesis. The enzyme that directs this is called RNA polymerase, which can also stall at the kink produced by thymine dimers. This can result in truncated mRNA templates, with potentially disastrous results if they end up being transcribed into partial-length proteins. There’s a lot that can go wrong with a cell thanks to a little UV light.
The Repair Squad
Ironically, though, the fact that thymine dimers can form so easily — some estimates are that 50 to 100 thymine dimers form every second human skin is exposed to sunlight, a tanning bed, or even the UV light needed to cure nail polish, it seems — may have been the evolutionary pressure needed to build the biochemical machinery needed to fix these lesions. A whole host of DNA repair enzymes, called photolyases, have evolved to fix thymine dimers and other radiation-induced damage to DNA, especially in plants, which are obviously constantly challenged by ultraviolet light. Photolyases are interesting because they’re literally solar-powered — they contain an “antenna complex” consisting of cofactors that can absorb light at the blue end of the spectrum and in turn transfer electrons into the dimers to break them apart.
Photolyases are evolutionarily ancient; they can be found in almost every organism stretching back to the earliest bacteria. Humans and most other mammals have evolved an additional repair pathway, called nucleotide excision repair, to deal with thymine dimers; essentially, it recognizes the backbone kink and enzymatically clips a section on either side out of the DNA strand, which is immediately filled in by a team of enzymes.
It’s easy to say that nothing good can come from either ionizing or non-ionizing radiation acting on biological tissue; just looking at the tracks left in bone by X-rays certainly supports that. But radiation damage, especially to DNA, is a double edge sword. Yes, most lesions that aren’t repaired can potentially cause problems, up to causing lethal cancers. But the damage caused by radiation has also been a major driver of the mutations that power evolution, and as such is pretty much responsible for what life has become over the last couple of billion years.
















> photons — gamma rays, X-rays, cosmic rays, and ultraviolet light — or particles > — alpha rays and beta rays
HUH? At screwl they taught us photons are particles.
The problem with school… Teaching things, correct or not, or thorough is unimportant
Pff! Bone holes make you run faster! Walk it off!
Speed holes!
This is hinted at above but to make it more explicit: a couple of our DNA polymerases have editing/repair capability and can excise and try to rebuild dimers or other damage, but this only works with single-strand damage. They rely on having the other strand to provide a template for the DNApoly to move along. A double-strand break is generally believed to be unrepairable. (Or at least this was what was taught when I was taking biochem/microbiology classes: this is an area of very active research.)
I have a kinda intense friend with a PhD in nuclear engineering who is a big advocate of the radiation hormesis theory, that since animals have far better DNA repair enzymes than bacteria (because our cell replication rate is extremely slow, we can afford the cost of carrying around lots of complex extra DNA, whereas bacteria’s primary competitive advantage is its short replication rate so they have the shortest DNA they can survive with, aggressively removing anything they don’t absolutely need) low-to-medium levels of DNA are, in his opinion, an advantage to us because they kill bacteria faster than us, and historically we’ve died much more often from complications from bacterial infections (particularly pneumonia) than from the long-term damage caused by ionizing radiation.
There’s also the fact that the DNA “code” itself has error correction and a kind of redundancy check, where multiple base pairs are used to encode a single amino acid. When the DNA is spliced back together again, one base pair may be different but that doesn’t change the meaning of the “codon”.
And we also have a lot of non-coding “junk” DNA that appears just to act as a buffer against damage, for a sacrificial target against oxidative damage and viruses etc.
“Junk” organs too. ;-)
https://time.com/4631305/appendicitis-appendix-gut-bacteria/
Is there a test that will show if you have been exposed to some type of high radiation? I came into very close contact with the things the Navy calls UAPs in 2012 and 2013. My skin turned red and kinda raised all over my body then few months later went to ER because it looked like blood coming out my skin and open sores forming all over my body. Most my body hair started falling off also and hair on my head got thinner. Er did test and said my iron was off the charts and they sent me to the cancer center. They did all kinds of test including DNA genetic tests for different diseases and they said nothing was found and they said it was a medical mystery with all the weird symptoms I was having. My whole body was breaking down and I could tell I was about to die. They started giving me phlebotomies to bring my iron down and that has helped alot. Still getting them till this day. Just would really like to know if it was some type of radiation I was exposed to. I filmed the things that got close to me and took pics of them so this is not a joke. Is there some types of test my doctors can perform that would show anything now?
A friend of my has hemochromatosis and it too requires blood draws to purge excess iron. He says it comes and goes.
They checked me for that with the genetic test and it was negative for the gene mutations for it. They said I have the symptoms of that plus all kinds of other strange symptoms that has nothing to do with that disease. I had 2 different doctors tell me I’m a medical mystery. Both where cancer and blood specialist. They thought I had some type of cancer first because I got so sick and the way my body broke down so quick.
Last I heard the Fed still haven’t determined a safe level of x-ray radiation exposure-which apparently they haven’t because there is no safe level. Thus, make sure you FLOSS 2X DAILY or your dentist may consequently say that to save what’s left of your teeth he/she needs to take “low exposure” x-rays.
That is misleading and inaccurate. Here is a page showing typical doses from procedures and mentioning background radiations, from this is shows a dental x-ray is 1/750th of annual background radiation 0.004msv for the x-ray, 3.0 msv for background radation https://www.epa.gov/radiation/how-much-radiation-am-i-exposed-when-i-get-medical-x-ray-procedure
They also have reports on the risk of different doses, appendix A here lists under <0.1msv to statistically negligible. https://www.epa.gov/sites/default/files/2015-05/documents/fgr14-2014.pdf
While x-rays are ionizing radiation and therefore any amount does affect the body, doses with medical procedures are effectively safe as the usually extremely small dose dose is statistically unsubstantial. Furthermore the benefit of the X-rays is usually far outweighs the very tiny risk of exposure.
Okay, I stand partially corrected. However, as I’m sure you’d agree, the precise number of exposures is important too. I see my dentist twice yearly for cleanings and inspections. A new SET of dental at least once yearly is standard practice, so that’s between 8 and 12 exposures. As for annual background radiation, I’ve read that this can vary depending on location, where radiation exposure is greater at higher elevations and/or proximity to radioactive rock and soil. In any case, it’s unfortunate there’s apparently not even been any attempt to develop non-DNA damaging imaging technologies. This may not worry those who are young and/or virtually disease free. But ultimately they and/or their loved ones will be in the opposite condition and there will be no other choice than to do numerous x-rays over the body and/or the brain to assure accurate diagnosis of a serious condition. But as I keep scolding my doctors A BETTER WAY SHOULD HAVE BEEN FOUND BY NOW!!
Stick you in an MRI.
Only with proper ear protection. But apparently medical societies caution against using MRI for all cases as it may provide inadequate imaging info. See the section here on Overuse. https://en.wikipedia.org/wiki/Magnetic_resonance_imaging In fact, I had to be diagnosed with a lower back condition last week and they insisted on using x-ray.
This is meant as as comment to the one below but it doesn’t have a “reply”..
There’s also terahertz imaging that doesn’t penetrate too far (a few mm) as it’s stopped by water molecules, so it has limited use. Also, there’s ultrasound imaging that has greatly advanced in recent years. I think some of the early experiments on radiation dosage was done to unsuspecting soldiers. In the sixties, it was discovered that x-ray equipment manufactures recommended higher doses to to “non-white” subjects, leading to a “ruckus”.
So assuming 12 dental x-rays, that is 0.016% of background yearly background radiation, about what you get in 12 hours.It sounds like you scold doctors about things you really don’t understand. They spend tons of money researching way to get better imaging. They already have non-dna damaging radiation Ultrasound and MRI are the two I know of that have practical use, but each form of imaging has its place. With all those technologies there is lots of R&D to improve resolution and lower the required radiation.
The difference is, you get the entire energy in one flash over a few milliseconds versus 12 hours. In the latter case, the cells have more time to repair any damage.
The raw energy involved is similar in scale to getting shot with an airsoft pellet gun. I always get a little bit of a tingle on my cheek when they take the x-ray.
Agreed! +1000
Another thing is you get the equivalent of an X-ray every time you fly on an airplane. There’s less atmosphere between you and the Sun when you fly.
I’ve been around people who get nervous running their carry-on luggage through the X-ray at the airport. That dose is actually less powerful than what YOU (and your carry-on) will get during a flight.
> the Fed still haven’t determined a safe level of x-ray radiation exposure
That’s because the potential risk from low level radiation is so small that it vanishes in the background noise. It can’t reliably be estimated where radiation exposure stops having an effect because people die, get cancers and other ailments, a hundred times over by other causes.
That is also saying, if there is a risk, and it’s on a scale of a thousand or ten thousand times smaller than whatever you’re experiencing anyways, does it matter?
My parents were born in the 50s and told me stories of going to department stores to look at their feet through “Shoe Fitting Fluoroscopes”. Its a wonder they haven’t died of some form of foot or bone cancer. I have to wonder at the dose levels over time of the salespeople. Granted most of the radiation was pointed at the feet back scatter and broken (or cheap) shielding had to be an issue.
They also talked about the first microwaves working with the doors open and standing there to watch water boil.
Its a wonder developed nations made it past the 1980s.
And the salespeople were the most at risk, since they assumedly always used the machines while they existed: https://en.wikipedia.org/wiki/Shoe-fitting_fluoroscope
questionable use of microwaves and ionizing radiation sources are nothing in comparison to all the persistent chemicals out there…
As creepy as it initially appears, we now seem to have microwaves with glass doors that you can see right through. Which also hopefully actually block the fairly long wavelength microwaves. At least you can measure that.
https://metamaterial.com/solutions/transparent-emi-shielding/
A nicely written article Dan! Your structure of a thymine dimer is incorrect though – the methyl groups don’t migrate (it is presumably a 2+2 electrocyclic addition) and should remain on the “opposite” side of the R group rather than next to it.
“[S]ome estimates are that 50 to 100 thymine dimers form every second human skin is exposed to sunlight, a tanning bed, or even the UV light needed to cure nail polish”
Exposure is technically damaging your skin, yes. Should you be mindful of avoiding intense and prolonged UV exposure? Yes. Does it have progressive and cumulative effects? Yes. Still live your life but these three listed exposures are not 1:1 exposure ratios to each other is the only point here.
How intense is the actual UV exposure? What is the actual wavelength? That matters as well. If it is sunlight, where on the Earth are you at the time? Is there cloud cover? What time of day and what season is the exposure? It can differ quite a bit, even day to day.
Obviously the more exposure the more of an impact that will have on your body. You get too close to a very high intensity UV bulb and you will get a very, very quick and very intense “sunburn”. Black lights can also be exposing you to some UV as well. Measuring these types of UV exposures is fairly easy to do and can show the actual UV exposure amount but don’t consider those three that are listed here as automatically identical in dose or how it impacts you either.
Just because it is UV is like saying it is DC power. Ok, great, but what is the voltage and what is the amperage and what is the resistance?
As a side note, it sounds like there is work being done to further study this in more depth as to how UV actually impacts your skin given somewhat similar studies done on the role of X-rays on collagen and so forth. Really interesting though also not really a surprise either.
https://www.nature.com/articles/s41467-022-34247-z
“X-rays are invaluable for imaging and sterilization of bones, yet the resulting ionization and primary radiation damage mechanisms are poorly understood.”
Published in December 2022! How is this still poorly understood?
Timely article as Germany is now helping the US to get a nuclear war started in Europe.
No, that was done by Russia, Putin. They should not have started the war in Ukraine.
what they mean is that alphas and betas have mass at rest / invariant mass. Photons have an invariant mass of 0, but still have relativistic mass – the one that you get out of E=mc².
It’s sloppy terminology. They could write “or **massive** particles – alpha rays and beta rays: that should clear it up and avoid the sloppiness