Beef soup! You’d normally expect it to be somewhere from reddish-brown to grey, depending on how well it was cooked and prepared. However, strangely, an assistant professor found the beef soup in their fridge had mysteriously turned blue. That spawned an investigation into the cause which is still ongoing.
[Dr. Elinne Becket] has earned her stripes in microbiology, but the blue soup astounded her. Despite her years of experience, she was unable to guess at the process or a source of contamination that could turn the soup blue. Indeed, very few natural foods are blue at all. Even blueberries themselves are more of a purple color. The case sparked enough interest that [Elinne] went back to the trash to collect photos and sample for research at the request of others.
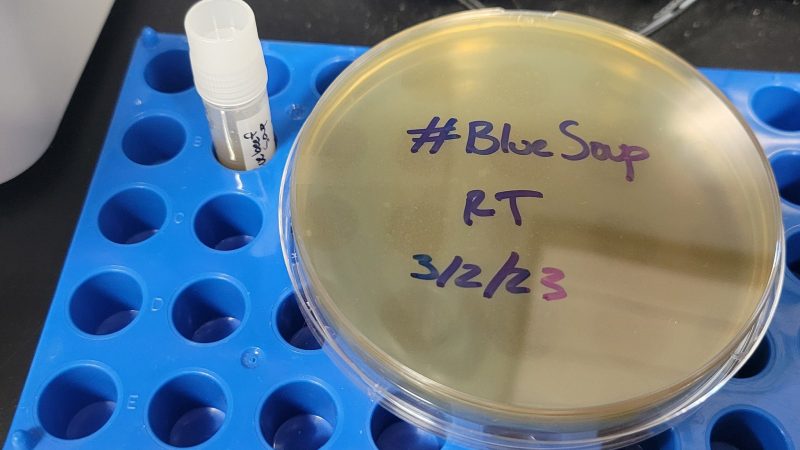
Thus far, metagenomic DNA analysis is ongoing and samples of the soup have been cultivated in petri dishes. Early analysis shows that some of the microbes form iridescent colonies, Another researcher is trying to determine if the bugs from the soup can make blue color appear on soft cheese. There’s some suspicion that a bacteria known as pseudomonas aeruginosa could be the cause of the blue color, but that presents its own problems. P. aeruginosa is classified as a Biosafety Level 2 pathogen which would require some researchers to abandon work on the project for safety reasons.
The jury’s still out on this microbiological mystery. If you’ve got some ideas on what could be going on, let us know in the comments!















Pseudomonas smells fruity and is instantly recognizable by smell alone. No advanced culture or sequencing work necessary.
That only leaves me with MORE questions – how on Earth did you learn that a biosafety level 2 contaminant ‘smells fruity’? Do you have a career that causes you to go around sniffing contaminants? Inquiring minds must know!
I don’t know about them, but I’ve dealt with a recurrent infection of it. Mine was a dayglo orange, though.
We streaked all sorts of stuff out during medical microbiology and then used the usual algorithms to identify the “unknown”. I got serraria which is bright red and was a chipshot. I have no idea why paeudomonas is BSL 2 because patients get pseudomonas infections all the time and they are not on any particularly aggressive isolation precautions. You have to be pretty sick and have “other stuff” going on usually but still. You can smell pseudomonas when you walk in the room usually. C diff too, unfortunately. So either we were and still are grossly unsafe with o it medical education or there is something else going on with the bio safety designation. Good question.
Agree. Except that a quick google seems to show that BSL2 handling is just about routine lab practice anyway. Pseudomonas growing on a petri dish is not regarded as a particular hazard. A new sample (like a swab or sputum sample) is often initially handled in a safety cabinet because at this early stage you don’t know what the specimen might contain (eg TB, or something else more serious for lab safety than Pseudomonas)
Experience as a medical microbiologist. Pseudomonas is a fairly common bacteria isolated from infections. Grown on certain media if forms characteristic colonies with iridescent sheen and scent.
There’s not a medical technologist in micro that doesn’t know what pseudo smells like. It’s very common and very distinctive.
Most of us would call it ‘fruity’ or ‘grapey’. I’m one of the less common ‘rubber/leather, like a shoe store’ types.
Umm. Red cabbage will turn substantially blue in an alkaline environment. It wouldn’t surprise me if other reddish pigments from plants did similarly. (Hmm. Not quite the shade of blue shown in the linked thread, though.)
This.
The blue color might not stem from a bacterial contamination itself, but from a change in pH (which itself may be provoked by a bacteria contamination), effectively turning reddish pigments into a blue color.
Many carotenoids and flavonoids change color depending on the acidity of the medium
Do you mean alkaline soil while it is growing or having the head put in an alkaline environment after it is picked?
Blue-green algae? it’s common enough, and there are variants that are bright blue.
Tags that will always hold only one article: bluesoup
Was this in Kentucky? “Blue soup of Kentucky, keep on…”
One child just didn’t like the soup / didn’t want to eat it again an threw in a vile of blue food coloring. ;-)
Vial… doh.
* colouring
+1
but the DSAian spelling kinda took over. :-/
Whoosh!
Yes! Here in Denmark it is a common practice to secretly add blue food coloring to various food at Christmas time and blame it on the gnomes :-)
This isn’t even the first time! I was surprised this particular incident was only at the beginning of the month, because I could have sworn I’d heard of another incident long before that. And I did find this: https://www.stuff.co.nz/taranaki-daily-news/news/10675415/Glowing-meat-to-stay-a-doggone-mystery
my 2 cents is that the soup had a dash of red wine and there was a change in PH
Like Spirulina? (Technically not algae but Cyanobacteria)
That was meant in reply to PWalsh, above…not sure why it didn’t nest beneath their comment…
Possibly a metal bring turned into nanoparticles by microorganisms. Fungi and bacteria synthesize metallic nanoparticles. Nanoparticles can have interesting interactions with light, providing some brilliant colors.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7150190/
https://www.hindawi.com/journals/jnm/2011/270974/
https://journals.asm.org/doi/10.1128/AEM.00264-10
Do a few simple tests.
What is the pH of a sample? If it is above 4ish then add something (as minimally reactive as possible) to reduce the pH to 3ish. If the color shifts towards a yellow you have a good chance of having some form of Phenol in the soup. Bromophenol is a common possibility that is used as an indicator that shifts from yellow to blue as pH rises from 3.0 towards 4.6.
Where it came from is the mystery. Microbiology isn’t my thing, so no help on that side, but applying Occam’s razor and the fact that Bromophenol is a very common lab indicator, the answer could be as simple as someone accidentally contaminated the soup with indicator solution.
It that turns out to be the case, your lab needs to have a safety discussion as soon as possible.
Otherwise, enjoy exploring the possibilities.
Starch Iodine complex?
Yeah that’s a simple test for conversion when making your own beer.
I have seen the refrigerators of some lazy people that had, what I observed to be, laboratory experiments being conducted in them. Advanced multi-year studies in mycology and bacteriology… possibly bioweapons, I did not take samples.
…or stay for dinner.
I once threw away a jar of chutney that was 12 years expired. I have to have moved it, then transferred it into a new fridge.
I told this story to my mom. She searched her pantry, found something more expired.
I kind of miss Spamcam.com. Perhaps it’s time to reboot it.
Simple, it wasn’t beef, it was Smurf meat.
Sometimes beef is sold as a marinated pack for a roast. I had the experience of including purple onions in the cooking and getting colorful blue roast ! The marinade contents listed carbonate so it was probably a pH effect on the onions. A bit daunting visually but still tasted great, no other effect.
Garlic is my bet! https://www.epicurious.com/expert-advice/why-does-garlic-turn-blue-article
Could it be a reaction from being cooked in a copper pot? , copper sulphate is very blue.
Maybe the soup was made from a left-over TV dinner — ZZ Top
I’ve had spoiled cooked rice turn a similar shade of blue. The culprit was most likely Chromobacterium subtsugae, used as a bioinsecticide in rice farming. If there’s any rice – or rice noodles – in this soup, something similar could be happening here.
Could be a chemical reaction with garlic.
I’ve seen it turn green and blue in various environments.