The gene-therapy-based treatment called Casgevy was recently approved in the UK, making it the first time that a treatment based on the CRISPR-Cas9 gene editing tool has been authorized for medical treatments. During the clinical trials, a number of patients were enrolled with either sickle cell disease (SCD) or β thalassemia, both of which are blood disorders that affect the production of healthy red blood cells. Of the 45 who enrolled for the SCD trial, 29 were evaluated in the initial 12-month efficacy assessment, with 28 of those found to be still free of the severe pain crises that characterizes SCD. For the β thalassemia trial, 42 patients were evaluated and 39 were still free of the need for red blood cell transfusions and iron chelation after the 12-month period, with the remaining three showing a marked reduction in the need for these.
Both of these blood disorders are inherited via recessive genes, meaning that in the case of SCD two abnormal copies of the β-globin (HBB) gene are required to trigger the disorder. For β thalassemia a person can be a carrier or have a variety of symptoms based on the nature of the two sets of mutated genes that involve the production of HbA (adult hemoglobin), with the severest form (β thalassemia major) requiring the patient to undergo regular transfusions. Both types of conditions have severe repercussions on overall health and longevity, with few individuals living to the age of 60.
The way that the Casgevy treatment works involves taking stem cells out of the bone marrow of the patient, after which the CRISPR-Cas9 tool is used to target the BCL11A gene and cut it out completely. This particular gene is instrumental in the switch from fetal γ globin (HBG1, HBG2) to adult β globin form. Effectively this modification causes the resulting cells to produce fetal-type hemoglobin (HbF) instead of adult HbA which would have the mutations involved in the blood disorder.
For the final step in the treatment, the modified stem cells have to be inserted back into the patient’s bone marrow, which requires another treatment to make the bone marrow susceptible to hosting the new cells. After this the patient will ideally be cured, as the stem cells produce new, HbF-producing cells that go on to create healthy hemoglobin. Although safety and costs (~US$2M per patient) considerations of such a CRISPR-Cas9 gene therapy may give pause, this has to be put against the prospect of 40-60 years of intensive symptom management.
Currently, the US FDA as well as the EU’s EMA are also looking at possibly approving the treatment, which might open the gates for similar gene-therapies.
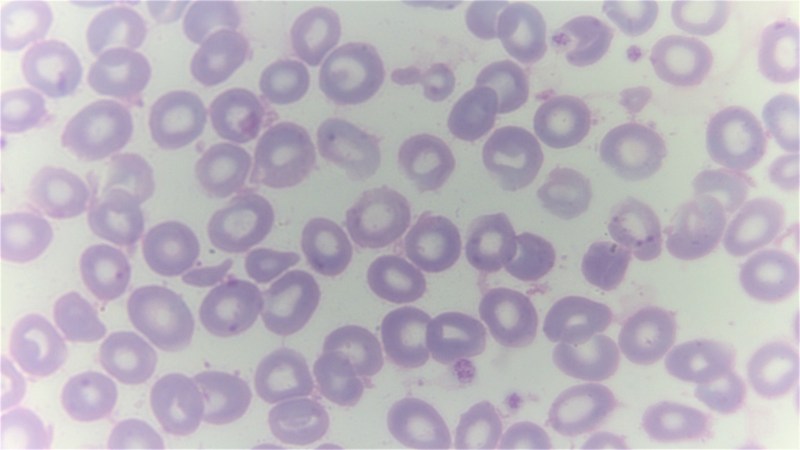
Top image: A giemsa stained blood smear from a person with beta thalassemia. Note the lack of coloring. (Credit: Dr Graham Beards, Wikimedia Commons)
















“another treatment to make the bone marrow susceptible to hosting the new cells.” Sounds a bit like chemo.
One of these days the costs will come down as the technique becomes more common place and used for even more genetic disorder issues. And as mentioned if you were to calculate how much a patient who makes it to 60 costs the healthcare system during their lives for just dealing with the disorders you’d likely find it a wash or less costly to carry out the treatment.
Quality of life should also be considered.
I had read about Sickle Cell treatments using gene therapy decades ago. I hope we finally get past the attempts and find ways to fix what we can to improve the life of those afflicted with such diseases.
Not all genetic disorders can be “cured” in the adult by the described method as suboptimal gene variants can change the developing embryo and child in ways that are irreversible.
Hacking the human genome….the ultimate hack?
Have they tested the blood of a treated patient against malaria? Don’t get me wrong. I wouldn’t choose to suffer from sickle cell disease just to be immune to malaria. But whether they lose that immunity or not is still an interesting question.
Malaria is both preventable and treatable so if they lose protection who cares, but you are right it would be an interesting study.
SCD doesn’t make one immune to malaria – just more resistant to it.
The trouble with this therapy is that the cost is in the millions of pounds per patient.
But experimentally trying something new on a small scale is always expensive. Just like hacking things is usually not a cheap option. Now they know it’s not just feasible, it’s allowed on a large scale, they can optimise the creation of the treatment.
The upfront cost is high but if successful it’s probably less expensive than decades of the current treatment. The UK healthcare system is mostly about minimizing cost not treating sick people.
I agree. This therapy will never succeed where it is needed most. To those comparing it to a lifetime of hospital bills- sure. In the US or UK. But compared to the zero dollars of hospital bills the vast majority of patients in developing nations get, (they get no treatment at all) it will always be out of reach.
As a comparison then only way the recently developed malaria vaccine can work is because it is about $3 a dose, now, and will only get cheaper.
Finally, and again sadly, sound financial decisions based on future savings just isn’t how decisions get made by any governments and this $2M per patient is well out of reach of NGOs and non profits and everything.
As a technology tho, hooray! While this may never succeed widely not will at least help our understanding of the disease and is a valuable research tool.
And even if they can’t make the treatment meaningfully cheaper you have to compare the ongoing and continued cost to the health care for these people – when it frequently costs some inpatient time that costs in the nurse/equipment and further costs (at least for nations with social medicine) for the loss of earning as the patient isn’t productive. I’d say it can quite easily get to good value for money if you turn somebody who either can’t work or who’s productivity is reduced into a ‘normal’ member of society you gain more in taxes and simultaneously eliminate an ongoing cost of their treatment that could have even can add up to bed blocking and grinding your healthcare system to a halt or forcing a greater expansion in capacity.
Though the ongoing question to treatments like this that only patch up that persons life has to be future costs – if you won’t allow Genetic modification to filter out these problems then your societies children are going to become ever more likely to need these sort of treatments later as all the folks that previously wouldn’t have children because of their problem whatever it was now might, and they can still pass the genetic faults on.
Genetic counseling would still be needed for these people.
Until they figure out how to genetically engineer sperm and/or eggs
I wouldn’t hold my breath for that
You dont need to work at the sperm or egg level.
Free from regulatory restrictions the simplest pathway is,
Skin cell → stem cell + (crispr or prime editing) → spermatid
ISCI that spermatid into the patners egg
TADA!
Chasing a recessive traits?
Do it again with your second genetic contributors line
You now have two modified spermatids,
Take one enucleated oocyte and ISCI both of those spermatids (DSC)
TADA!
Ethics boards, eugenics fears, and regulatory bureaucracy are bigger obstacles than the science at this point
Your second paragraph makes a more general point that few think about: medicine in general is dysgenic. Left to his own devices, Darwin does a pretty good job of keeping the species healthy as a whole.
Meant to reply to someone but this garabge comment system can’t even stack replies. Get your shit together hackaday.
Only if you assume that all weakness SHOULD be fatal.
People who carry the sickle cell gene are more resistant to malaria. The same is true for many other disorders that require two copies of recessive genes; simply being a carrier has benefits.
Nature, in the Darwinian sense, doesn’t care how a species survives. We got brain, we developed medicine, we benefit as a species because of those things. That makes the species “more fit” so we survive.
Quin there is a massive difference between fatal and the illness actively preventing or the risk of passing this unpleasant condition on to your children meaning you electively will not have (as many) children. Which ends up as a big difference between actively in effect breeding in these issues – which then turns this problem up to 11 for the nations/individuals healthcare budgets in future.
Its absolutely a tricky thing to balance, as you could easily go to far and end up with a lack of useful gene variety and the Aryan race type ideologies.