Chances are good that most of us will go through life without ever having to perform gas chromatography, and if we do have the occasion to do so, it’ll likely be on a professional basis using a somewhat expensive commercial instrument. That doesn’t mean you can’t roll your own gas chromatograph, though, and if you make a few compromises, it’s not even all that expensive.
At its heart, gas chromatography is pretty simple; it’s just selectively retarding the movement of a gas phase using a solid matrix and measuring the physical or chemical properties of the separated components of the gas as they pass through the system. That’s exactly what [Markus Bindhammer] has accomplished here, in about the simplest way possible. Gas chromatographs generally use a carrier gas such as helium to move the sample through the system. However, since that’s expensive stuff, [Markus] decided to use room air as the carrier.
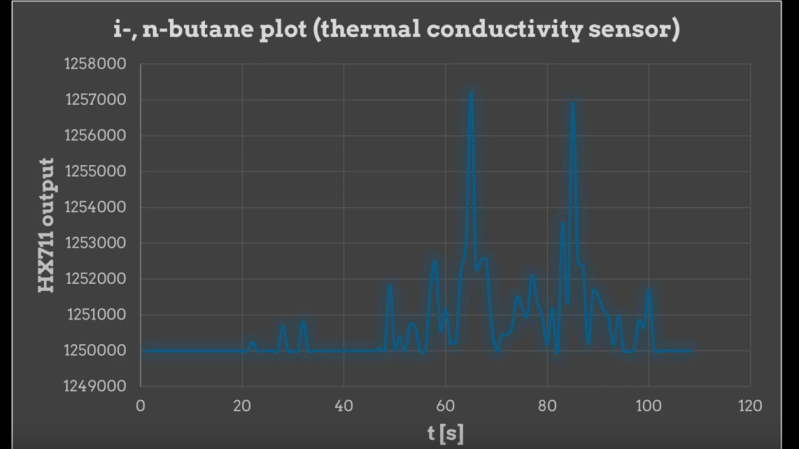
The column itself is just a meter or so of silicone tubing packed with chromatography-grade silica gel, which is probably the most expensive thing on the BOM. It also includes an injection port homebrewed from brass compression fittings and some machined acrylic blocks. Those hold the detectors, an MQ-2 gas sensor module, and a thermal conductivity sensor fashioned from the filament of a grain-of-wheat incandescent lamp. To read the sensors and control the air pump, [Markus] employs an Arduino Uno, which unfortunately doesn’t have great resolution on its analog-to-digital converter. To fix that, he used the ubiquitous HX7111 load cell amplifier to read the output from the thermal conductivity sensor.
After purging the column and warming up the sensors, [Markus] injected a sample of lighter fuel and exported the data to Excel. The MQ-2 clearly shows two fractions coming off the column, which makes sense for the mix of propane and butane in the lighter fuel. You can also see two peaks in the thermal conductivity data from a different fuel containing only butane, corresponding to the two different isomers of the four-carbon alkane.
[Markus] has been on a bit of a tear lately; just last week, we featured his photochromic memristor and, before that, his all-in-one electrochemistry lab.















That’s really cool. Going to guess though, that standard reference mixes will cost a lot more than the whole thing :-/
Packing the silica into a stainless steel column would enable you to put the column into an oven to enable doing chromatography on heavier hydrocarbon mixtures. Even get crazy and control the oven to do a temperature gradient, which could allow even more complex mixes to be detected by the thermal conductivity detector (which is what I had doing this with the capillary GC back in the 80’s when I was a young lab rat. )
Thank you. I am planning an oven with PID controller for the next version.
Does the silica gel need to be kiln dried regulary? perhaps after each use? I don’t know much about chromatography grade silica gel, but those moisture absorbant packs seem to need regular drying…
Amazing build and documentation ! That was a pleasure to watch. Any differences in performance between the thermal conductivity sensor and MQ-2 when used on the same gas mixture ? Is one more suitable than the other for certain gases ?
It depends. The MQ-2 cannot decide between n- i-butane, the thermal conductivity sensor can.
I recall a chem lab manual from the 70s that showed how to construct a GC. If memory serves the stationary phase was Tide detergent.
I have a book from the 70s called „Laborbücher Chemie, Adalbert Wollrab, Gaschromatographie“ were a home made gas chromatograph is described.
This may have been a reprint from Scientific American. They had a regular column(sic) called “The Amateur Scientist” and the september 1967 issue was .. a gas chromatograph that used Tide detergent and an incandescent bulb as the sensor. The article is available, but its behind a paywall.
However, today we have readily available silica gel, and a computer that costs a few dollars!
Here you can read at least the first page: https://www.jstor.org/stable/24930971
Would be quiet interesting as they describe a homemade FID. If anyone has access to the complete 7 pages and willing to share, please let me know.
The newer scientific American amateur scientist book has a great chapter on building a thin layer chromo using a soap dish and agar.
Can this be used to find the level of CO2 in the air?
A SCD-40 true CO2 sensor would be more suitable for a single gas detection I guess.
You’d be hard pressed to find a matrix that selectively retards the different gases in air, so no, not really.
Wow. This is really fantastic DIY project. And resonance with 35 years ago similar school lab project on 8-bit home computer.
What about a mixture of molecular sieves? One could use a thermal gradient if need be, much like an hplc uses solvent gradients.
Great build and the use of the hx-711 was brilliant for the thermal conductivity detector. A few rules of thumb to improve performance: To improve the peak separation and sharpness use a smaller diameter column and try to minimize all dead volume from the injector through the detector. To improve sensitivity of the thermal conductivity cell, increase the thermal conductivity of the block by substituting aluminum for the acrylic. The difference in thermal conductivity between the carrier gas and whatever you’re analyzing also contributes to the sensitivity. Hydrogen has the highest thermal conductivity and will give the best sensitivity if you want to deal with the generation and flammability issues. Switching over to nitrogen eliminates the problem of oxygen burning up the tungsten filament and the filament can be run hotter which also increases the sensitivity. Again, awesome build! love seeing analytical tools here on hackaday. Would love to see you tackle an FID detector.
Thanks for all the gret input. A flame ionization detector is definitly on my list:)
I have 20+ years as an Analytical Chemist. I loved the robustness of a Flame Ionization Detector. It fit our broad needs. I highly recommend a stainless steel column and an oven and run it around 300F. Use zero grade gasses like your nitrogen, hydrogen and air. @Markus Bindhammer, hit me up if I can be of any assistance. I look forward to your progress.
using the data from the graph were you able to calculate an area count of each peak?