Hackaday has a long-running series on Mining and Refining, that tracks elements of interest on the human-made road from rocks to riches. What author Dan Maloney doesn’t address in that series is the natural history that comes before the mine. You can’t just plunk down a copper mine or start squeezing oil from any old stone, after all: first, you need ore. Ore has to come from somewhere. In this series, we’re going to get down and dirty into the geology of ore-forming processes to find out from wither come the rocks that hold our elements of interest.
What’s In an Ore?
Though we’re going to be talking about Planetary Science in this series, we should recognize the irony that “ore” is a word without any real scientific meaning. What distinguishes ore from other rock is its utility to human industry: it has elements or compounds, like gems, that we want, and that we think we can get out economically. That changes over time, and one generation’s “rock” can be another generation’s “ore deposits”. For example, these days prospectors are chasing copper in porphyry deposits at concentrations as low as 1000 ppm (0.1%) that simply were not economic in previous decades. The difference? Improvements in mining and refining, as well as a rise in the price of copper.

There’s a story everyone tells in my region, about a street in Kirkland Lake, Ontario that had been paved using waste rock from one of the local gold mines and then torn up when the price of gold rose enough to reprocess the pavement a part-per-million of microscopic flakes of yellow metal. That story is apocryphal: history records that there was mine product accidentally used in road works, but it does not seem it has ever been deemed economic to dig it back up. (Or if it was, there’s no written record of it I could find.)
It is established fact that they did drain and reprocess 20th century tailings ponds from Kirkland Lake’s gold mines, however. Tailings are, by definition, what you leave behind when concentrating the ore. How did the tailings become ore? When somebody wanted to process them, because it had become economic to do so.
It’s similar across the board. “Aluminum ore” was a meaningless phrase until the 1860s; before that, Aluminum was a curiosity of a metal extracted in laboratories. Even now, the concentration of aluminum in its main ore, Bauxite, is lower than some aluminum silicate rocks– but we can’t get aluminum out of silicate rock economically. Bauxite, we can. Bauxite, thus, is the ore, and concentration be damned.
So, there are two things needed for a rock to be an ore: an element must be concentrated to a high enough level, and it be in a form that we can extract it economically. No wonder, then, that almost all of the planet’s crust doesn’t meet the criteria– and that that will hold on every rocky body in the solar system.
Blame Archimedes
It’s not the planetary crusts’ fault; blame instead Archimedes and Sir Issac Newton. Rocky crusts, you see, are much depleted in metals because of those two– or, rather, the physical laws they are associated with. To understand, we have to go back, way back, to the formation of the solar system.

There’s a primitive elemental abundance in the solid bodies that first coalesced out of the protoplanetary disk around a young Sol and our crust is depleted in metals compared to it. The reason is simple: as unaltered bodies accreted to form larger objects, the collisions released a great deal of energy, causing the future planetoid to melt, and stay molten. Heat rejection isn’t easy in the thermos vacuum of space, after all. Something planetoid sized could stay molten long enough for gravity to start acting on its constituent elements.
Like a very slow centrifuge, the heavier elements sunk and the lighter ones rose by Archimedes principle. That’s where almost all of Earth’s metals are to this day: in the core. Even the Moon has an iron core thanks to this process of differentiation.
In some ways, you can consider this the first ore-forming process, though geologists don’t yet count planetary differentiation on their lists of such. If we ever start to mine the nickel-iron asteroids, they’ll have to change their tune, though: those metallic space-rocks are fragments of the core of destroyed planetoids, concentrated chunks of metal created by differentiation. That’s also where most of the metal in the Earth’s crust and upper mantle is supposed to have come from, during the Late Heavy Bombardment.
Thank the LHB

The Late Heavy Bombardment is exactly what it sounds like: a period in the history of this solar system 3.8 to 4.1 billion years ago that saw an uncommonly elevated number of impacts on inner solar system objects like the Earth, Moon, and Mars. Most of our evidence for this event comes from the Moon, in the form of isotopic dating of lunar rocks brought back by the Apollo missions, but the topography of Mars and what little geologic record we have on Earth are consistent with the theory. Not all of these impactors were differentiated: many are likely to have been comets, but those still had the primordial abundance of metals. Even cometary impacts, then, would have served to enrich the planet’s crust and upper mantle in metals.
Is that the story, then? Metal ores on Earth are the remnants of the Late Heavy Bombardment? In a word: No. Yes, those impacts probably brought metals back to the lithosphere of this planet, but there are very few rocks of that age left on the surface of this planet, and none of them are ore-bearing. There has been a lot of geology since the LHB– not just on Earth, but on other worlds like the Moon and Mars, too. Just like the ore bodies here on Earth, any ore we find elsewhere is likely to be from other processes.

One thing that seems nearly universal on rocky bodies is volcanism, and the so-called magmatic ore-forming processes are among the easiest to understand, so we’ll start there.
Igneous rocks are rocks formed of magma — or lava, if it cools on surface. Since all the good stuff is down below, and there are slow convection currents in the Earth’s mantle, it stands to reason some material might make its way up. Yet no one is mining the lava fields of Hawaii or Iceland– it’s not just a matter of magma = metals. Usually some geochemical processes has to happen to that magma in order to enrich it, and those are the magmatic ore forming processes, with one exception.
Magmatic Ore Formation: Kimberlite Pipes
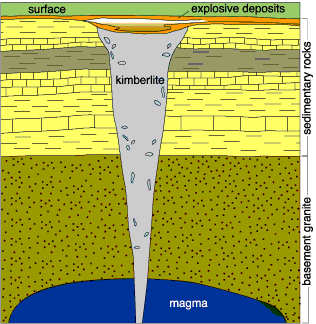
Kimberlite pipes are formations of ultramaphic (very high in Magnesium) rock that explode upwards from the mantle, creating vertical, carrot-shaped pipes. The olivine that is the main rock type in these pipes isn’t a desirable magnesium ore because it’s too hard to refine.
What’s interesting economically is what is often brought to surface in these pipes: diamonds, and occasionally gold. Diamonds can only form under the intense pressures beneath the Earth’s crust, so the volcanic process that created kimberlite pipes are our main source of them. (Though not all pipes contain diamonds, as many a prospector has discovered to their disappointment.)
The kimberlite pipes seem to differ from ordinary vulcanism both due to the composition of the rock — ultramaphic rocks from relatively deep in the mantle — and the speed of that rock’s ascent at up to 400 m/s. Diamonds aren’t stable in magma at low pressures, so the magma that makes up a kimberlite pipe must erupt very quickly (in geologic terms) from the depths. The hypothesis is that these are a form of mantle plume.
A different mantle plume is believed to drive volcanism in Hawaii, but that plume expresses itself as steady stream and contains no diamonds. Hawaii’s lava creates basalt, less magnesium-rich rocks than olivine, and come from a shallower strata of the Earth’s mantle. Geochemically, the rocks in Hawaii are very similar to the oceanic crust that the mantle plume is pushing through. Kimberlite pipes, on the other hand, have only been found in ancient continental crusts, though no one seems entirely sure why.

The great shield volcanoes on Mars show that mantle plumes have occurred on that planet, and there’s no reason to suppose kimberlite-type eruptions could not have occurred there as well. While some of the diamond-creating carbon in the Earth’s mantle comes from subducted carbonate rocks, some of it seems to be primordial to the mantle.
It is thus not unreasonable to suppose that there may be some small diamond deposits on Mars, if anyone ever goes to look. Venus, too, though it’s doubtful anyone will ever go digging to check. The moon, on the other hand, lacks the pressure gradients required for diamond formation even if it does have vulcanism. What the moon likely does posses (along with the three terrestrial planets) is another type of ore body: layered igneous intrusions.
A Delicious Cake of Rock

Layered igneous intrusions are, as the name suggests, layered. They aren’t always associated with ore bodies, but when they are, they’re big names like Stillwater (USA) and Bushveld (South Africa). The principle of ore formation is pretty simple: magma in underground chambers undergoes a slow cooling that causes it to fractionate into layers of similar minerals.
Fractional crystallization also has its role to play in concentrating minerals: as the melt cools, it’s natural that some compounds will have higher melting points and freeze out first. These crystals may sink to the bottom of the melt chamber or float to the top, depending on their density relative to the surrounding lava. Like the process of differentiation writ in miniature, heavy minerals sink to the bottom and light ones float to the top, concentrating minerals by density and creating the eponymous layers. Multiple flows of lava can create layers upon layers upon layers of the same, or similar, stacks of minerals.
There’s really no reason to suspect that this ore formation process should not be possible on any terrestrial planet: all one needs is a rich magma and slow cooling. Layered igneous intrusions are a major source of chromium, mainly in the form of Chromatite, an iron-chromium-oxide, but also economically important sources of iron, nickel, copper and platinum group elements (PGEs) amongst other metals. If nickel, copper, or PGEs are present in this kind of deposit, if they’re going to be economically extractable, it will be in the form of a sulfide. So-called sulfide melt deposits can coexist within layered igneous intrusions (as at Bushveld, where they produce a notable fraction of the world’s nickel) or as stand-alone deposits.
When Magma Met Sulfur
One of the problems with igneous rocks from a miner’s perspective is that they’re too chemically stable. Take olivine: it’s chock full of magnesium you cannot extract. If you want an an easily-refined ore, rarely do you look at silicate rock first. Igneous rocks, though, even when ultramafic like in Kimberlite pipes or layered melt deposits, are still silicates.
There’s an easy way to get ore from a magma: just add sulfur. Sulfur pulls metals out of the melt to create sulfide minerals, which are both very concentrated sources of metals and, equally importantly, very easy to refine. Sulfide melt deposits are some of the most economically important ones on this planet, and there’s no reason to think we couldn’t find them elsewhere. (The moon isn’t terribly depleted in sulfur.)

Have you heard of the Siberian Traps? That was a series of volcanoes that produced a flood basalt, like the lunar mare. The volcanoes of the Siberian Traps were a primary cause of the End-Perimian mass extinction, and they put out somewhere between two and four million cubic kilometers of rock. Most of that rock is worthless basalt Most, except in Norilsk.
The difference? In Norilsk, there was enough sulfur in the melt, thanks to existing sedimentary rocks, to pull metals out of the melt. 250 million years after it cooled, this became Eurasia’s greatest source of Nickel and Platinum Group Elements, with tonnes and tonnes of copper brought to surface as a bonus.
Norilk’s great rival in the Cold War was Sudbury, Canada– another sulfide melt deposit, this one believed to be associated with the meteorite impact that created the Sudbury Basin. The titanic impact that created the basin also melted a great deal of rock, and as it cooled, terrestrial sulfur combined with metals that had existed in the base rock, and any brought down in the impactor, to freeze out of the melt as sulfides.

While some have called Sudbury “humanity’s first asteroid mine”, it’s a combination of sulfur and magma that created the ore body; there is little evidence to suggest the impactor was itself a nickel-iron asteroid. Once the source of the vast majority of the world’s nickel, peaking at over 80% before WWI, Sudbury remains the largest hard-rock mining centre in North America, and one of the largest in the world, on the weight of all that sulfide.
Since the Moon does not seem to be terribly depleted in sulfur, and has more flood basalt and impact craters than you can shake a stick at, it’s a fairly safe bet that if anyone ever tries to mine metals on Luna, they will be sulfide melt deposits. There’s no reason not to expect Mars to posses its fair share as well.














That was a very interesting article
Thank you! There will be more to come; hopefully you enjoy the rest of the series.
Actually, it’s not about iron or sulphur. Moon will be used commercially by US-based space contractors because it contains exponentially large deposits of Helium-3 which is needed for atomic energy and nuclear bombs. Russia and China are also interested but I doubt they can compete when faced with increasing economic sanctions.
Well it’s not currently used in any energy or weapons applications right now… We’re still pretty far from any kind of aneutronic fusion. That’s pretty advanced stuff. We’d love to be able to sustain any kind of fusion at all, and the H in H-bomb doesn’t stand for helium.
But the moon has 3-10x higher concentrations of a ton of different elements and minerals compared to the Earth’s crust, not just helium-3. Just about any rare earth is a lot less rare there. It’s a very large initial investment, though.
He-3 might be a big driver of lunar mining… someday. Like TG says, the technology to use it does not exist. No market means that the He-3 bearing rigoliths cannot yet be called an ore. Maybe someday, but not today.
Old prospectors would say that you’d have to find yellow ground (as in sulfur) to mark the location of a Kimberlite pipe at the surface. Great article!
I’m a big fan of this type of articles (mining and refining, field guides etc) and look forward to the coming installments of this one!
I do realize that they generally fall in the not-a-hack category, but I am very willing to let that slide as long as they are well-written and informative. Taking things apart to see how they work is great fun; lacking an active ore-forming region nearby to disassemble, reading about them is a good substitute.
Mining&refining not-a-hack?
Since when? ;)
Great article! Also, I went to high school in Temiskaming Shores/New Liskeard…it’s not often I come across someone in Northern Ontario!
Really interesting read, thanks for that. For quite a long time I have been mulling over the fact that (metallic) ores could be leftovers of ancient impacts, thanks for clarifying that they may not (directly) be. Can’t wait for the next instalment of this series.