With the availability of increasingly cheaper equipment, welding has become far more accessible these days. While this is definitely a plus, it also comes with the elephant-sized asterisk that as with any tool you absolutely must take into account basic safety precautions for yourself and others. This extends to the way you prepare metal for welding, with [Dr. Bernard], AKA [ChubbyEmu] recently joining forces with [styropyro] to highlight the risks of cleaning metal with brake cleaner prior to welding.
Much like with common household chemicals used for cleaning, such as bleach and ammonia, improper use of these can produce e.g. chlorine gas, which while harmful is generally not lethal. Things get much more serious with brake cleaner, containing tetrachloroethylene. As explained in the video, getting brake cleaner on a rusty part to clean it and then exposing it to the intensive energies of the welding process suffices to create phosgene.
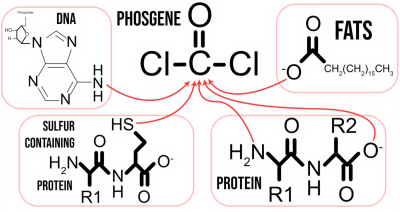
Used as a devastating chemical weapon during World War I, phosgene does not dissolve or otherwise noticeably reduce in potency after it enters the lungs. Instead it clings to surfaces where it attacks and destroys proteins and DNA until the affected person typically dies from disruption of the lung’s blood-air barrier and subsequent pulmonary edema. Effectively your lungs fill with liquid, your blood oxygen saturation drops and at some point your body calls it quits.
The video is based on a real case study, where in 1982 a previously healthy 23-year old man accidentally inhaled phosgene, was admitted to the ER before being rushed to the ICU. Over the course of six days he deteriorated, developed a fever and passed away after his heart stopped pumping properly due to ventricular fibrillation.
Basically, if you are off minding your own business and suddenly smell something like musty hay or freshly cut grass when nobody is mowing the lawn, there’s a chance you just inhaled phosgene. Unlike in the video, where the victim keeps welding and waits a long time before going to the ER, immediate treatment can at least give you a shot at recovery if the exposure was mild enough.
As with laser safety, prevention is the best way to stay healthy. In the case of welding it’s essential to fully cover up your skin as there is intense UV radiation from the work area, protect your eyes with a quality welding mask and ideally wear a respirator especially when welding indoors. Show your eyes, lungs and skin how much you love them by taking good care of them — and please don’t use brake cleaner to prep parts for welding.
















“and please don’t use brake cleaner to prep parts for welding.”
Non-chlorinated brake cleaner is safe.
Better safe than sorry, who knows the lies cheap companies put on their products.
+1
It’s all fun and games ignoring the “don’t use brake cleaner” advice. Until you grab a can of older brake cleaner by accident…
Brake cleaner of any variety is inherently very evaporative. That’s its purpose. I’m wondering how he was welding in a puddle of brake cleaner in the first place? That was not explained. I think would be very difficult to duplicate his accident.
Like a lipo pack the size of a stick of gum claiming to be 10 bazillion mAh? Dishonest companies will try to sell things using inferior material to make easy money. Never use any chemical on metal about to be welded even if it claims to be chlorine free cleaner. Sand it off the old fashioned way.
Brake cleaner isn’t going to do anything for rust anyhow.
Somebody trying to weld over rust should take a single GD class or ask the worst welder he knows for some tips.
What did they do, buy a welder at home depot?
But not an angle grinder, gloves or hat?
Using safety squints?
Whole family watching him and his new toy?
Like the YouTuber backyard patio casting aluminum in flip-flops and shorts.
Telling his toddler to stay back 5 feet (can’t be too safe!)
Not editing that out of his vids, makes me suspect ‘video troll’.
But I think he was just full-idiot who caught CPS’s eye, channel disappeared. Digressing.
A lot of the non chlorinated brake cleaner is just acetone in an aerosol can.
Do not weld on galvanized metal as it also produces toxic products that can kill you.
It probably won’t kill you. It just makes you wish it had.
It’s no fun at all. Been there, when I was much younger and stupider.
It takes surprisingly little exposure: I was three days in bed from a single 8-inch bead connecting galvanized pipe to some 1/2″ plate for an expedient snow plow repair. I thought I could get away with that small a weld in a closed garage, because it was cold outside.
Was mich nicht umbringt, macht mich stärker (“What does not kill me makes me stronger” Or at least a little smarter.)
It isn’t uncommon for gavl to contain Cadmium. That will be worse than a bad day.
That said, zinc galv, in and of itself, rarely causes major or lasting harm. But when it does, it is bad.
Exposure to Zinc ‘Smoke’, I know as ‘Zinc Chills’.. Slight Fever like Symptoms.. and ‘Date Night’ is off the Table for a Few Days, as ‘Performance’ will be Non-Existent.. :(
How do I know?..
Cap
Pretreat w swimming pool acid to remove galvanized layer.
Well ventilated area, but not stupid dangerous, zinc end’s in solution.
Wth is swimming pool acid and does it have anything to do with chlorine because that sounds like a bad idea.
Some places think it’s not stupid to use muriatic acid (hydrochloric acid, HCl) to adjust the pH of swimming pool water. And some people, ignorant of the actual name, just call it ‘swimming pool acid’, to the mild horror of other places.
By the time it’s diluted down in pool water, it doesn’t result in a pH low enough to evolve (much) free chlorine.
The corresponding pH boost is, naturally, pure lye, NaOH.
The result of recklessly combining the two is a nice saltwater pool :-)
It’s worth noting that the case study is not from welding, but some other source of “concentrated gas”.
With tetrachloroethylene being quite volatile, you’d have to be very quick to have enough of it still on the surface and close enough to the welding to produce enough phosgene. It seems you would need at least a milliliter of reaction mass to exceed the 0.2 ppm exposure limit in 5 m³ of air.
Of course worth avoiding, but again a youtube video that attempts to make things scarier than they practically are.
Yup.
It’s a theoretical hazard when brazing refrigeration lines too, but I didn’t think it happens too often.
Ok. Cool. I certainly don’t WANT it to be true that it would be that easy to mess up and die. But what are you talking about? The video said it was from welding parts coated in brake cleaner. I get part of what you are saying, I’ve used brake cleaner and I know it dries quickly. But are you saying the video is lying? Or that they were mistaken? Ok, cool. Can I have some evidence before I go mix brake cleaner and welding please? Or a little clarification if that’s not what you meant?
Well, yes and no.
The vapour pressure at 20C is only about 14mmHg (<2KPa), but it does tend to evaporate quickly in well ventilated spaces. In a poorly ventilates space, esp. on a rough, rusty surface, it can take quite a while to evaporate. I will guess that might have been the issue with the case described, but that is only a guess.
Then comes the issue of removing the vapour from the area once the liquid is off of the surface.
It is a rare, but brutal, event that someone gets enough phosg. to cause harm while welding. But sometimes all of the holes in the swiss cheese line up. The easy way to avoid this is don’t use chlorinated cleaners when prepping a weld area.
If you DO use a chlorinated cleaner intentionally, you are taking a heavy risk. I do not recommend at any time. Horrible way to die.
If you accidentally use it, time and ventilation are needed, probably more time and more ventilation than you think, ESPECIALLY with a rusty surface. Also, low, flameless/arcless heat will increase the vapour pressure (about 380mmHg/50KPa at 100C) will greatly speed the evaporation. Also keep in mind that the density of the vapour is much higher than air, so it can collect in low areas of poorly ventilated spaces, like low areas of a fuel tank. You always need more ventilation than you need. In a fuel tank, for example, AFTER there is no liquid left on the surface (none. Not a drop. Not a film. All of the cleaner has evaporated) a MINIMUM of ten complete air changes, insuring no dead spots, would be a starting point.
Brake cleaner is one of the few good chemicals we can still get in California. It works for cleaning out the straws for expanding foam insulation, which otherwise get clogged after first use and you gotta toss the foam, no matter how much you have left. Great stuff, brake cleaner (see what I did there?)
If you mean the non-chlorinated kind, then yeah – but that’s not what most people think of as brake cleaner. Certainly not the originality formulation, which has been banned in California for ages.
I was surprised to see so much about phosgene gas without mention of its use in chemical warfare.
So… let’s say I did clean something with brake cleaner and then wanted to weld it. Would simply waiting for it to dry and working outside be good enough?
Is it common to weld in an enclosed space? I think my garage would fill up with fumes in less than 10 seconds if I did that. I at least have the garage door open if I weld and that’s with no extra chemicals. But then, I barely weld and don’t have much experience either.
Thinks like this where you can get a lethal dose before you even know you are being harmed are scary.
Depends on what you weld and how clean it is. Back in the days when I did arc welding I would have the door open and ventilation going at full speed. Nowadays I only TIG weld and I don’t bother with either.
TIG is a form of arc welding just fyi.
Was this added to the article after your comment: “Used as a devastating chemical weapon during World War I, phosgene does not dissolve or otherwise noticeably reduce in potency after it enters the lungs.” ?
It’s difficult to gas shield weld in the wind, especially gusty wind.
If it’s really blowing and you have to weld, you’re likely to have to compromise somehow.
Flow to 11, then use vehicle/coworker/shop dog as wind break (joke…welding shops should not have dogs/cats).
I buy brake cleaner for bike parts at Action shop each time I go, it was 2EUR for a bootle:
https://www.action.com/fr-be/p/2509405/nettoyant-pour-freins-c-c/
I wear gloves now, because it also penetrates in the skin.
This is incomparable to the stuff they get in the USA. What we get is flamable. It is essentially ether and hexanes just like starting spray which is not benign but still much less harmful. You can weld around ‘our’ brake cleaner and the stuff will just catch fire if it somehow remains on your parts.
The USA stuff is chlorinated solvents. Non flamable but much more toxic.
This! I have never seen the chlorinated non flammable stuff here in Germany. I guess it’s not even allowed in the EU for exactly those reasons (very carcinogenic). But I read that some states in the US of A already phased the chlorinated stuff out and many will follow.
Chloroform is allowed in EU.
Is StyroPyro okay?
yes
They made sure to point that out in the video
Pretty sure chlorinated brake cleaner has been linked to Parkinson’s just by itself. I just avoid anything related to chlorinated solvents, I don’t even like using shoe goo.
Like most of these things, Shoe goo is fine unless you live in California. Says so on the tube: “known to cause cancer in California”. Not sure if using it here causes cancer for people in California or if you have to actually use it in Cali to cause cancer, but as I live the other side of the world, I’m safe.
/s, in case anyone is too slow…
They really need to improve the wording on those warnings.
They really need to improve on the wording on the propositions they put to vote, too.
Prop. 65 ranks pretty high on the “unintended consequences” list. Right up there with the Paperwork Reduction Act.
According to the prop labels, EVERYTHING in California causes cancer.
Because Prop 65 covers SO MANY chemicals, and the list changes all the time, many companies that might have one of those chemicals in a product just choose to put the warning label on everything they make. CYA and all that…
Reminds me of seeing some popcorn from John Deere in a promotion online. Literally everything they sell has the Prop 65 label, as a rule, to protect them from lawsuits, and to skip the cost of testing.
I prefer some other sites like Harbor Freight, where they break out each Prop 65 labeled item with the trigger, like lead, solvents, BPA, etc (no thanks to some prior litigation), so you at least have some idea what’s in there.
There are no minimums for Prop65. That’s the problem. If it’s large an enough amount to be detected at all, it gets labelled. Even if it’s thousands of times smaller than a toxic amount. Don’t imagine that it was just an oversight. When moneyed chemical corporations couldn’t de-rail the bill, they put in this flaw which makes it worse than useless. Call it a conspiracy theory, but I don’t think it was accidental.
CRC makes two kinds flammable and nonflammable. the video refers to the non flammable which is chlorinated. A great way to avoid this is to purchase the flammable kind. It’s the only kind I use.
The flammable kind seems to work better too.
If you smell fresh mowed hay, Run Away!
I used to work for a company that made PVC conveyor belts. As a degreaser we used perchloroethylene.
One day my manager asked what would happen when that stuff caught fire.
Me , with a couple of years of chemistry under my belt and an unhealthy interest in all humanicides , immediately answered PHOSGENE!!
Blank stare in return.
You know , that stuff they used in WW1 to off each other.
The coin dropped , and the 4 barrels were banished to the great outside the same day.
Fun fact : the production temperature of PVC belts is just 15 degrees under the ignition temperature of PVC. One F-up and they carch fire. Only half a year after I left the poop hit the fan and the place burned to the ground.
Mostly because the fire brigade was smart and evacuated everybody in a half mile radius , including themselves.
I’ve had this page bookmarked for so long I can’t even remember. https://www.brewracingframes.com/safety-alert-brake-cleaner–phosgene-gas.html
This happened at my workplace, late 1990’s. A welder collapsed and in the hospital, they attributed his poisoning to Brakleen and the arc UV, as I remember.
Note- Brake Clean formula varies depending on the country, and even the era. Back then it was particularly nasty like flux removers. Many mechanics I imagine living a shorter life due to using this nasty stuff.
Ha! I wasn’t remembering wrong then!
Back in the mid-’80s I worked in a factory where they used trichloroethylene to clean/de-grease metal parts after turning. It was in hot baths. Someone told me that if it got too hot, it would produce phosgene gas.
Since then, I have always used acetone and IPA to clean metal.
Best use for the over-hopped hipster slop.
Guinness Extra Stout is better after being used to clean metal.
You’re probably thinking of vapor phase degreasing. The solvent is in an open top heated chamber that has a cooling jacket (water or refrigerated) further up around the perimeter. The solvent boils and creates solvent vapor, which condenses back to liquid when it comes into contact with anything cooler, including the cooling jacket. This results in a tank with boiling solvent in the bottom and a “cloud” of solvent vapor than never gets above the cooling jacket. The solvent vapor is much heavier than air so does not rise up out of the top. The vapor solvent is always clean, as it has just evaporated, so parts lowered into the vapor cloud are rinsed with continually clean solvent even if the solvent in the bottom had oils or other dirt. Cool parts will instantly cause large amounts of solvent to condense from the vapor cloud and drip into the boiling pool below. The parts will be heated by this and as they were slowly raised above the vapor cloud any remaining solvent almost immediately evaporated, leaving the parts clean and dry when removed. Periodically, a solvent recovery cycle was done that raised the temperature just above the solvent boiling point, and captured the condensing vapors into a seperate tank, and the leftover liquid was only oils and dirt which was drained and disposed of. In theory, very little solvent was used up as it could just be continually reused. Parts never were immersed in contaminated solvent, just the pure vapor. This was widely used in many industries like electronics (like PCBs and chip wafers) and metal machining (especially after machining operations that used oil for cooling/lubrication). Freon (liquid variants), perclor, triclor, and methyl chloride were common solvents. These solvents were not flammable, but had toxicity risks, and the nasty decomposition to phosgene if contacting very high temperatures (like a flame). These systems were significantly curtailed because it was difficult make them meet VOC limits, and the low-cost solved used were discontinued. Vapor phase cleaning is still used in some industries (like chip wafers) with high quality equipment and modern (safer but expensive) solvents as it’s difficult to reproduce the high level of cleaning this method gives.
When I was small my dad had an old box of glass carbon tetrachloride fire extinguisher “bombs” you were supposed throw at a fire. 😳
Luckily we never used them.
But there was one missing from the case. I wonder if someone used it.
I’ve seen this come up a few times on welding forums and the like, every time I’ve looked into it it seems very much like we don’t get the “good” stuff here in Europe anyway.
The brake cleaner here does not seem to have trichloroethylene but the warnings on it seem to suggest what is used is even worse.
Also says you should not expose the can to more than 50 degrees Celsius, so you better not keep it in a truck/car in summer then.
I wonder what came first, brake cleaner or the weapon, maybe some soldier who used to be a mechanic said ‘I hate these enemies so much, I’m about ready to throw brake cleaner at them!’
Looking at some of the videos Styropyro has on YouTube I think his demise will be while playing with the high energy stuff he builds. His latest handheld laser has “Bad idea” written all over it.
As a pyromaniac, I have planned out my final thought.
It will be: ‘At least I’m not on fire.’
In high-school shop class, circa 1980, we were very slightly supervised. Sometimes instructor left for long periods. We thought it was the funniest thing ever to make big slag piles in the welding booth. And to melt/burn stuff with the electric arc welder. Didn’t learn welding then and there (taught myself years later), but i did learn how to make slugs for vending machines, a tool to jimmy a door and a dust explosion with sawdust from below the table saw. God, I’m so glad to have grown up then!
This is rather timely – tonight I was watching a couple of videos like this – about using a homemade carbon-rod DC welder, a homemade “flux”, and using it all together to weld aluminum:
https://www.youtube.com/watch?v=sjj1LsiJMTI
If you don’t find this one (in case the creator took it down) – there are plenty other examples out there.
Anyhow – the “flux” is being made by reacting zinc with toilet bowl cleaner that has a large percentage of hydrochloric acid in it – which then forms a solution of (likely?) zinc chloride and hydrochloric acid, which is then “painted” on the part as a “flux” – which mostly instantly boils away.
Lotsa chlorine atoms there – and heating that up will make phosgene…at least in theory. I’m not willing to test it – maybe someone here is a chemist or has better knowledge than I do? I know brake cleaner has different stuff in it than toilet bowl cleaner, but from what am aware of, unless I’m really wrong, heating up just about anything with chloride atoms in it can be trouble…
I also find it odd that this Hackaday posting showed up…but that’s coincidence for ya!
An update – found this reddit thread:
https://www.reddit.com/r/metalworking/comments/5m1pct/never_weld_over_brake_cleaner_it_will_kill_you/
It had a link to here – more interesting info:
http://weldingweb.com/showthread.php?182941-DANGER-using-BrakeCleaner-to-clean-your-Parts
Also – in all the above “mix” – there was mention of toilet bowl cleaner being dangerous as well, so I guess I wasn’t being too paranoid…that said, I also read some stuff that it has more to do with the emitted UV (check) than the heat. Also something about “organic solvents” or such, and not “salts” by themselves (but then, if you heat table salt up enough…well, somebody mentioned road salt, so again…chlorides…).
Also – another suggestion found in comments on this: If your cleaner says “non-flammable”, then don’t use it around heat or extreme UV sources (aka welding) – and that even if you let it dry and it seems dry and all evaporated…it may not be!
If you must use a cleaner, use acetone (but note: very flammable!) – and if you must use a non-flammable cleaner, do a “final rinse” with acetone.
Apparently, organic chlorinated chemicals are what makes it all “non-flammable”.
Also – crazy enough, there are plenty of other possible similar risky things – like halon systems – that can also generate phosgene, as well as how a fluid used in the past (for all kinds of things – including, get this: as a fire extinguisher) called “carbon tetrachloride” – there’s that chlorine thing again – with the added “tetra-” which always also pops up in the “Things I won’t work with” blog that was around or still around – though for different reasons, of course. Still, you start stacking those things up like that (yeah, let’s make it 4x worse!) – well…
And with that comment, you can instantly tell I’m no chemist – lol.