[youtube=https://www.youtube.com/watch?v=yKigsN8046k&w=580]
We’ve mentioned that it’s hard to find someone not selling or crowd funding something at Maker Faire. Despite the fact that [Ryan Edwards] is selling his boards, we still got the feeling that he’s a hacker who is selling just to make sure the idea he had is available for other hackers to use. He showed us his interface boards for inexpensive pH probes.
Since we’re always looking for more chemistry hacks to run, it was nice to hear [Ryan’s] description on how these probes (which can be had for around $9 on eBay) actually work. It turns out it’s all about salt. When it comes to the electronics, the board provides a connector for the probe on one edge, and pins for voltage, ground, and I2C on another. Rig this up with your microcontroller of choice and you’ll be building your own automatic pool doser, fish tank minder, or one of a multitude of food-related hacks.
Head on over to Sparky’s Widgets to see a few other demo applications.

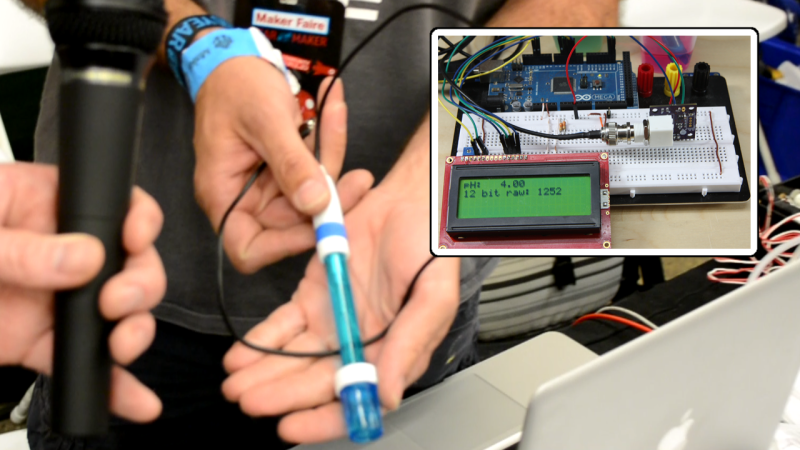














crazy i ran across his site recently. interesting stuff over there gotta give him some props and definitely worth checking out
Thanks for the kind words and checking out my site really I appreciate it, I wish the site and documentation were in a bit better order but its always a WIP, I am trying to get some manuals up and update the eC, IsoIon and other pages with the most current information. I will have a writeup on DIY eC probes soon just finalizing the process a bit, the materials are still a PIA as usually with these things.
Hi, I have just bought an e/c interface board from you. Is there a cheap probe on ebay that you recommend? It will be immersed in a hydroponic nutrient solution. Thanks!
Great little writeup on i2c isolation on his site too.
Glad you found it useful,it seemed a natural fit for this type of problem instead of more traditional opto isolation (I really wanted single supply for simplicity, but control more stuff downstream i.e digipot or PGA etc…), although some closed source makers have picked up my methods sigh.
have a link?
Been waiting for this for years despite moderators negating that thought.
Are these the probes he is making or you need some additional probes for it?
Use any standard PH probe ($5 and up…). Sparky’s widgets for PH are essentially a op-amp with an ADC. You use the Nerst equation to work out the PH value (or work out if the probe needs replacing).
I found this to be one of the best reads on the subject: http://www.hach.com/asset-get.download.jsa?id=7639984488
The only off the shelf MCU solutions for this seem to be from atlas-scientific and Sparky. I’ve got a couple of the Sparky’s miniPH sensors which seem to work great.
His explanation of how a pH probe works inaccurate.The pdf Squeeks posted is an excellent resource if you don’t want to spend $100 bucks on an electrochemistry text(or even if you do).
Leaving a glass probe in your experiments, as suggested in the article, is a terrible practice. At best you are adding electrode solution to the pond/beer/whatever and degrading the fritted glass, in turn shortening the lifespan of the probe. At worst biofouling will clog the pores of the glass and salt bridge requiring you to soak the probe in cleaner, which may or may not work, either way drastically decreasing the lifespan of the probe.
Lastly, pH and other ISE, probes are notoriously bad at holding calibration. To have any meaningful numbers coming out of the meter you need to calibrate every 6-12 hours depending on use and environment. There are a few lab-on-a-chip style probes(Durafet by Honeywell) that are ‘no calibration’ and can be left in place but they are a few hundred dollars even on auction sites.
I don’t know about the durability and stability of the very cheap sensors available in aliexpress: http://s.click.aliexpress.com/e/37AqZ7I