While many people have tried their hand at anodizing aluminum at home, there are plenty who would just as soon leave it up to the professionals due to the highly concentrated sulfuric acid required for the process. [Ken] started thinking about the process and wondered if there was a way to get comparable results using chemicals that are easier to obtain and dispose of.
Through some experimentation he found that sodium bisulfate (NaHSO4), which is a sodium salt of sulfuric acid, can easily be used in its place with great results. The chemical is typically advertised in hardware and pool stores as “Aqua Chem”, and can be had at a very reasonable price. When paired with the proper DC current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready for coloring with RIT, readily available cloth dye.
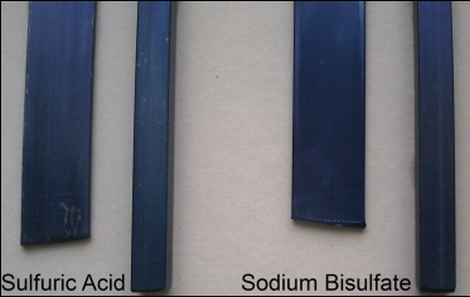
We were impressed with the results, and when looking at [Ken’s] test pieces, it seems that the metal dyed with sodium bisulfate has a more uniform, less streaky coloring to it. It’s also worth mentioning that [Ken] has found it is fairly easy to etch the aluminum before anodizing using a solution of sodium hydroxide, which is great for individuals who prefer a more matte finish.
If this is something that interests you, be sure to swing by his site. He has a posted nice video overview of the process that may be of some help.















Actually Mike it is NaHSO4. One too many H’s LOL, Ken.
I think that should be NaHSO4, not HaHSO4.
Titanium can be anodized much more easily:
Some diet coke, an adjustable power supply, and depending on the voltage you get different colors. (try at around 20-30V DC) It’s fun to do!
Don’t drink the Coke afterwards though.
http://en.wikipedia.org/wiki/Sodium_bisulfite = NaHSO3
Wrong link. The chemical used here is sodium bisulfate, NaHSO4.
Little Willie was a chemist.
Little Willie is no more.
For what he thought was H2O,
Was H2SO4.
–David Smillie
In school ours was:
Johnny was a chemist’s son,
but Johnny is no more,
for what he thought was H2O
was H2SO4.
Apparently there’s a shirt of that!
http://www.thinkgeek.com/tshirts-apparel/unisex/sciencemath/7d73/
Two guys walk into a bar.
First guy says, “I’ll have some H two O.”
Second guy says, “Sounds good. I’ll have some H two O, too.”
Second guy died. The end.
RIT, readily available cloth dye. That tends to give really poor results in many colors.
“highly concentrated sulfuric acic” is actually about 50:50 battery acid (which is already diluted) and water. It’s about 25 – 30% sulfuric.
“can be had at a very reasonable price” same as sulfuric.
BTW, when your’e life is ending, you’re dying. When you alter color with dye, you’re dyeing.
Thank you for noticing, that one annoyed the hell out of me too lol.
I guess it depends on if you are doing this in an enclosed space with no ventilation… then I think both are appropriate.
Also note, if sodium bisulfite and nitric acid (sometimes used as a desmut/deox) are mixed, sulfur dioxide (a poisonous gas) is produced.
finally! someone who really knows what they’re talking about, and not just educated at Youtube-University.
“youtube-university” is totally anachronistic in 2011.
When I was working at AT&T (building robot systems), I had to make a lot of those large button type panels, you know the type, “STOP” “START” etc….
The buttons came with a aluminum plate that fit around the button and provided a rectangle at the top for the name of the button.
The idea is, you engrave the button, then dip it in this (provided) chemical. And the normal oxidation on the plate would prevent the chemical from acting on it.
The chemical then “anodized” (as they called it) the newly engraved portion of the plate. Resulting in very nice looking lables, with a permanent coloration.
I keep finding, and them promptly forgetting this company and the chemicals. I think the chemicals were custom. Not off the shelf stuff like this. Kinda pricey, but not too expensive.
If I can find the chemical/company, I’ll post it here. You could do a whole aluminum front panel at one time, and then “etch” it with this stuff and have some really sharp looking front panels.
Maybe a little late, but I think I know what you are talking about.
It’s called “Alodine” (it’s a solution of chromates) and usually gives a golden tint to the object being threated.
“A guideline is 2.8 to 10 amps for one square foot of aluminum. This process is very open to experiment and optimization.”
Most commercial type II anodizing is done at 12 ASF (amps per sq ft).
“Two ideas that I have not tried yet is to raise the temperature of the anodizing solution, and the thought that it would be helpful to find a material to resist anodizing solution attack.”
This is unlikely to work. Commercial anodizing is done at 70 F or lower. In fact, you use chillers to maintain temp when doing more than say 30A.
Sodium Bisulfate is basically half neutralized Sulfuric Acid in powdered form. Some of its advantages include safety, evironmental and shipping concerns. Sodium bisufate is much safer than liquid acids. OSHA classifies sodium bisulfate as an irritant, sulfuric acid is classified as corrosive. The NFPA (National Fire Protection Association) Hazard rating for sodium bisulfate is 1-0-1, for sulfuric acid it is 3-0-2. This corresponds to sulfuric acid having serious health effects and sodium bisulfate having slight health effects. For employee safety concerns, the product that contains sodium bisufate would be prefered.
If the products are spilled, sodium bisulfate can be swept up and put back into a container with no enviromental problems to deal with. If a liquid acid is spilled it will contaminate the ground or get into the sewer or creek and cause serious environmetal problems.
When shipping the two products DOT classifies sodium bisulfate as Non-regulated, with no shipping requirements. The liquid acids are classified as corrosive, therefore, they have extra shipping costs and additional packaging requirements.
“it seems that the metal dyed with sodium bisulfate has a more uniform, less streaky coloring to it”
You cannot draw any meaningful conclusions from this single set of photos with unknown process parameters.
I wouldn’t scrub with steel wool due to iron contamination. A light sanding with aluminum oxide sand paper and a detergent wash should be good. Avoid soap with anything for “soft hands” as that will contaminate the aluminum.
You might want to add a small amount of liquid glass (sodium silicate) to the caustic etch as this will help prevent smutting (a black film that results from over etching). Just be sure to use a hot water rinse afterwards. If you have some, EDTA or other chelating agent will help limit sludging of the solution.
If the aluminum is in good condition, you actually shouldn’t need to mechanically prep it. A good detergent wash and etch should be all you need.
(Sorry. I did conversion coatings and cleaners for aluminum and steel for 11 years.)
Why specify a hot water rinse after sodium silicate / caustic etch? Does cold not work somehow?
I work with etching high power electrical contacts. In my experience, hot water rinse always works better than cold. I use sodium nitrite heated to between 615 and 700 deg F. Cold water does almost squat for a rinse. Room temp (75F) water works a little better. 100+ d/f works best.
Same idea as sugar dissolving in hot water better/faster than cold water. More ‘energy’ available.
Hot water helps dissolve any salts that may be present on the surface of the part. This isn’t to say you can’t adequately rinse with cool or cold. It’s just typically faster/easier to rinse with hot water.
how about using methanol. I heard that would strip the oxide from AL. Figure since you are an expert I would ask.
Methanol also causes blindness in surprisingly low doses and possibly even death and doesn’t even strip aluminum. Caustic soda (lye) does. It’s alkaline though so contact precautions are in order.
Methanol is only dangerous if ingested. I think 15 mLs (pure) will cause blindness. As little as 30 mLs for death.
As pointed out, however, methanol will not etch aluminum. It might slightly solvent clean the surface, but it won’t remove oxide. You need a good caustic solution for that or a good acidic etch (nitric with a small amount of either HF or NaF to increase the attack).
Leave the methanol for your fondue pot.
You might want to add a small amount of liquid glass (sodium silicate) to the caustic etch as this will help prevent smutting (a black film that results from over etching). Just be sure to use a hot water rinse afterwards.
Can you elaborate on this a bit more?
A straight caustic etch (particularly a fresh bath) can really eat into aluminum. Sodium silicate is a kind of inhibitor that helps slow this down. As with all solutions, it’s a matter of time, temperature, and concentration. A low concentration etch may not be a problem, but if you’re finding that you’re having to really, really etch in order to properly prepare the entire surface, the inhibitor helps prevent overetching in the areas that clean off more easily. You want a hot rinse to help remove any inhibitor.
The smutting is a black film that forms on the aluminum. You can generally remove it by wiping it away with a rag.
If the aluminum is in decent shape, an inhibitor may not be needed as you won’t have to do too much etching.
Something else to keep in mind is that surface finishing is as much art as science. What works on one alloy in one set of conditions may not work on another. YMMV.
Interesting stuff.
Although the lazy and the cheap usually find silvery aluminum sufficiently attractive.
Aqua Chem is actually a brand name that encompasses a number of pool chemicals. Any store that sells pool chemicals will have sodium bisulphate. It’s labeled either pH Decreaser, pH Minus, or Dry Acid and comes in many brands.
I would like to see tests to prove it’s anodized and not just “Fake anodizing”. Anodized aluminum has a stronger surface to resist abrasion and damage, strike it with a hammer compared to a raw piece.
Otherwise I’ll stick to transparent paint and clearcoat to give me a better finish.
^ Second this!
If it takes dye, it’s anodized. Period. Might be only .5 mils if the voltage or current was low or such but you definitely grew an oxide layer if it takes the dye.
Placed on my list of thing to try, but may have to wait until Spring 2012. My shop doesn’t have heat, therefor no running water. I think something one desires to have available when working with corrosive liquids. Nice find Mike…
I once managed to do anodiz(s)ing perfectly at home, produced a load of beautiful parts. Every try since then has resulted in horrible un-dyed, greyed, etched surfaces and no luck. I’ve given up.
I did this a few years ago making armor. Except, I used vinegar, it’s much cheaper. A friend also used lye.
will this also work with ammonium persulphate ?
When I used the reverse process to remove rust from steel, I had to clean the anode each time. Do you have to clean the aluminum cathode each time? Also, can you use tin/lead as the cathode instead of pure lead? I have plenty of that and can cast it to any shape.