If you can’t answer the riddle, don’t feel bad. Metal conductors usually conduct electricity and heat. Usually, that’s true, but researchers at the Department of Energy’s Lawrence Berkeley National Laboratory and at the University of California, Berkeley, have found that vanadium dioxide can conduct electricity without conducting heat.
The Wiedemann-Franz Law states that good conductors of electricity are also good conductors of heat. Vanadium dioxide not only switches from an insulator to a conductor at 67C (152F), but it appears that it also doesn’t conduct as much heat as that law predicts while it is in its electrically conductive phase.
There are a few other materials that can conduct electricity better than heat, but these only operate at temperatures well below zero degrees. The researchers found that heat transfer attributable to random motion of electrons in a normal conductor is reduced in vanadium dioxide because the electrons move in a regular pattern like a moving fluid instead of randomly moving like particles.
By adding different materials to vanadium dioxide, the researchers were able to reduce the temperature that the material becomes a conductor as well as increase its ability to conduct heat. This allows tuning the material for specific uses.
In addition to providing new insights into how materials work, researchers hope the material will bring about advances in recovering waste heat and converting it to energy. This same team has been finding interesting uses for the exotic material, including using it as a micro robotic muscle (see video, below).
We’ve covered some of Berkeley’s exotic material antics before. We also check in on the national laboratories from time to time.
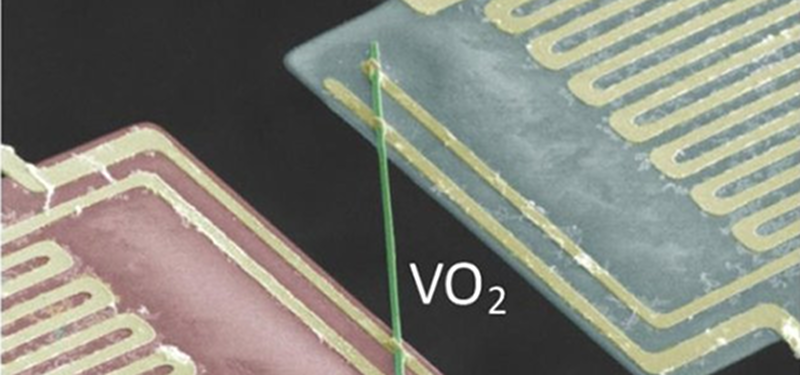
Photo credit: Junqiao Wu/Berkeley Lab












can conduct electricity and conducting it well are two different things. The article is very vague about is it as good as Gold when it conducts? or is it closer to what steel does?
I worked with VO2 thin films before and the sheet resistance I would often measure in metallic phase was about 1kohm/sq for 100 nm films. this would be about 0.1 mOhm*m resistivity. Not quite comparable to “good” conductors but the other properties of the material would probably still make it desirable in certain applications.
Another interesting property that VO2 has that was not mentioned in the article is that it’s optical properties change a lot in the infrared frequencies after the phase transition without significantly changing its properties in the visible frequencies. Good films of VO2 can block nearly all infrared light passing through when heated to its conducting state whereas in the semiconducting state, around 60% of the infrared light can pass. This would be a good property for smart windows that block the entry of infrared light from the sun once the room is at an appropriate temperature without changing the visible light significantly.
You specify the behavior of IR but not if it is translucent to visible light at all.
Isn’t VO₂ a ceramic rather than a metal?
I think technically it is an inorganic. The original article says metal though. Vanadium itself is a metal. I am not sure exactly what makes a material qualify as a ceramic other than inorganic and non metallic.
Are not all metals found a oxides in nature? We generally don’t consider iron ore to be “metal” in she strictest sense, though we do classify meteors as stony or metallic based on composition.
Iron oxide isn’t a metal, it’s a metal oxide semi-conductor. Part of what defines metals is the ability to conduct heat and electricity. Following that come ductility and/or malleability. At the atomic level you can refine the definition to the ways in which a metal can bond & all the ‘sea of electrons’ analogies.
The reason we call meteors metallic, is because they have native metals as their main composition. Most of the metal oxides found are restricted to those areas exposed to earth’s atmosphere and weathering.
Actually I’m pretty sure the definition of metals has something to do with their property of having a shared electron cloud instead of nice discreet orbitals. It’s also part of why metal has a really low heat capacity for it’s weight: the electrons get jiggled instead of the atoms themselves so the particles moving have less mass. If Vanadium Oxide does something similar at temperature it would then be described as a metal at that temperature and a semiconductor otherwise. Sorta like the idea of metallic hydrogen.
Well, it doesn’t have carbon, so it’s definitely “inorganic” in the chemistry sense. Metal vs. ceramic is pretty much how dominant metallic bonding is vs. covalent bonding in making up the structure. So it’s entirely possible that what’s going on here is a transition from a state with lots of covalent bonding to a state with more metallic bonding at specific temperatures. That is, at low temperatures the relatively few collisions don’t allow the vanadium oxide to lose the valence electrons, and you form a covalent matrix. At higher temperatures, the electrons break free and you get more of a metallic structure.
The weak thermal conductivity is weird, though. My “physicist, but not a solid-state physicist or chemist” guess would be like you have some restricted region that you’ve got a metal-like electron cloud, and outside of that you’ve got a more ceramic-structure. The free electron region would conduct like a metal, and the ceramic structure wouldn’t conduct heat well, unlike a normal metal where the bonding is metallic throughout. I’d have to read the paper though.
It should be noted that making electrical connections that don’t conduct heat well on a PCB (thermal vias) would be something like this: you restrict conduction to a small thin trace for a short bit to impede heat flow but have little effect on the overall electrical resistance. Not a great analogy, obviously, but might be similar.
I guess you’re right. Vanadium is a metal. Vadium Oxide is material with ceramic properties, inorganic non electric- and heat conducting, depending on temprature?
I think VO₂ is the exception to the rule.
Wikipedia:
“A ceramic is an inorganic, nonmetallic[a] solid material comprising metal, nonmetal or metalloid atoms primarily held in ionic and covalent bonds.”
“Varying crystallinity and electron consumption in the ionic and covalent bonds cause most ceramic materials to be good thermal and electrical insulators”
https://en.wikipedia.org/wiki/Ceramic
Yes, it seemed a bit like cheating to me too when they answered “What metal conducts electricity, but not heat?” with a ceramic instead of a metal. Although a conductive ceramic is quite an interesting material in and of itself. Most oxides aren’t very good conductors of either heat or electricity.
actually sometimes it gives the right information but not all the time. it’s kind of bad but then not that bad
VO2 is definitely a ceramic and not a metal. Because the Vanadium has oxidationstate +IV and not 0 (as it would be in a Vanadium-metal.)
Things are not defined so much by what they are but how their current configuration behaves, https://en.wikipedia.org/wiki/Nonmetal#Allotropes
Could be useful in temperature sensors to prevent the rest of the system warming up the sensor through wiring, although maybe on a (severely?) limited range.
Higher resolution thermal cameras maybe.
There isn’t a metal that doesn’t conduct heat.
FTFA: “doesn’t conduct as much heat as” . So it DOES conduct heat, it just doesn’t fit the law FOR METALS.
Hydrogen can be a metal
Aluminum is a metal
Iron is a metal
Vanadium is a metal
Water (H2O) or Aluminum oxide or Iron oxide (rust) are not metals. Neither is Vanadium oxide.
For starters, metals have to be elements, alloys of elements, not counting additives or impurities. Their oxides are not metals, they are metallic oxides.
Attention-whoring by scientists who know better
The word “metal” has lots of uses.
Astronomers use “metal” to mean anything other than hydrogen or helium.
Chemists use “metal” to mean a specific group of elements (basically everything except the upper-right corner of the periodic table).
Solid-state physicists use “metal” to mean a material with a conduction band below the Fermi energy, or a material with a resistivity that increases with temperature (these two are related).
The scientists here are using the last definition, which fits vanadium dioxide above its critical temperature.
“… will bring about advances in recovering waste heat and converting it to energy”
i hate to be that guy, but heat is already energy ;-)
maybe you want to write something like converting it to other forms of energy, like electrical energy
Well, granted…useful energy instead of waste…
The Wiedemann-Franz law doesn’t apply to diamond, which (in the opposite way to the material here discussed) is a good conductor of heat but a very strong insulator:
https://en.wikipedia.org/wiki/Material_properties_of_diamond#Electrical_properties
it would seem that that shouldn’t be a law but a rule of thumb instead, if there are exceptions to natural law it usually means we have misunderstood something fundamental.
The Wiedemann-Franz law applies to electrical conductors. It doesn’t go the other way.
Lots of things conduct heat, but not electricity. It’s the other way that’s really hard.
If it’s a conductor of electricity, but not of heat, what happens to the heat produced by the electricity conducting through it?
It produces no heat because it is a room-temperature superconductor, obviously.
Well, not quite room-temp, but close… But shhhh, don’t spoil it for the researchers!
It radiates? Or convects. They’re your 2 options, I think.
or deforms I suppose.
When I ask myself if the amazing future that science fiction stories describe will happen, I have mixed feelings. On one had the physics behind space travel is discouraging. Radiation outside our protective magnetic field make travel to even Mars deadly and Einsteinian limits on travel make travel to stars an impossible dream.
On the other hand, there’s a lot in material science that offers amazing hope for improving life on earth. Nanomaterials and lossless electrical conductors are two examples This odd metal is another. What might we do with this odd metal?
Generation ships.
Smith&Wesson uses, or used Vanadian in their aluminum 1911series pistols some years ago. It made aluminum as strong as steel they advertised.
If we populate Mars and maybe some of the moons of Saturn or something then we can at least have space battles as the inevitable conflicts arise.
Vanadium dioxide isn’t a metal. Please change the clickbait title, it makes science hurt.
Interesting article though!
Vanadium dioxide undergoes what’s called a semiconductor-to-metal (or insulator-to-metal) transition at a critical temperature. Above that temperature, its conduction band has states below the Fermi energy, so it’s a metal. Below that, it’s a semiconductor or insulator, depending on your definition of the terms (only thing that separates an insulator from a semiconductor is the band gap size).
Interesting article though!
It´s a click-bait article from a science news. Not for HaD. It´s probably just there to compensate the post-electoral slow-down of traffic on HaD.
There are science news like this almost everyday. About solar energy, battery technology, nanotechnology….
Those breakthroughs are (yet) limited to laboratory, and maybe it would be wise to fence such articles out of HaD, where notorious HaD bloggers will at best copy-paste without really understanding the topic, or trash those with their ignorance.
Leave cutting-edge science articles to scientists !
How is silver oxide as a heat conductor? It conducts electricity. Tektronix used it on contacts because both silver and its oxide conduct.
I’m surprised. Silver was a verboten material ANYWHERE in our high rel system designs
I don’t consider this a metal, it’s more of a ceramic.
Metal has a specific definition, which vanadium dioxide satisfies above a certain temperature.
That would depend whose definition you’re using, right? It may conduct electricity above the phase transition temperature, but it presumably loses this property when molten, nor can it be drawn into wires or hammered into sheets (both typical characteristics of metals).
Yeah, that’s the chemical definition of metal. They’re using the solid-state definition, which is the location of the conduction band.
It also fits the astronomer’s definition of a metal, too. :)
So you have to heat it up to make it conduct, but it doesn’t conduct heat that much? Just having fun with it, I’m sure this will have an application.
Titanium has a relatively low thermal conductivity, as far as common metals go… about a third of iron’s. I can cook with my titanium spork without a problem.
Stainless steel is generally a poor thermal conductor but a good electrical conductor. In the opposite direction, sapphire is a relatively cheap good thermal conductor that is an electrical insulator.