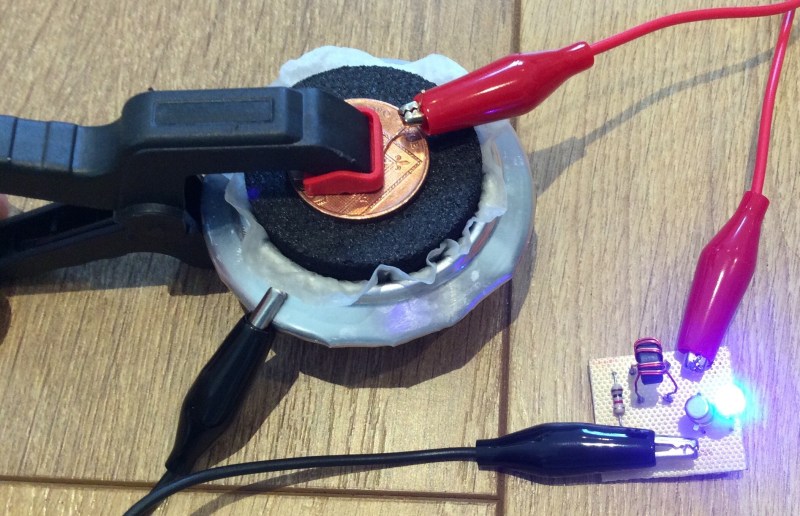
While batteries are cheap and readily obtainable today, sometimes it’s still fun to mess around with their less-common manifestations. Experimenting with a few configurations, Hackaday.io user [will.stevens] has assembled an aluminium-air battery and combined it with a joule thief to light an LED.
To build the air battery, soak an activated charcoal puck — from a water filter, for example — in salt-saturated water while you cut the base off an aluminium can. A circle of tissue paper — also saturated with the salt water — is pressed between the bare charcoal disk and the can, taking care not to rip the paper, and topped off with a penny and a bit of wire. Once clamped together, the reaction is able to power an LED via a simple joule thief.
 The cell averages an output of five milliwatts — at approximately 10% efficiently — which decreases as the salty water is used in the reaction and just plain dries up. Watering the battery daily — there’s an oddity — maintains the reaction and after a week you can see how much of the can has eroded in the process.
The cell averages an output of five milliwatts — at approximately 10% efficiently — which decreases as the salty water is used in the reaction and just plain dries up. Watering the battery daily — there’s an oddity — maintains the reaction and after a week you can see how much of the can has eroded in the process.
You can also use one light source to power other lights with joule thieves, or even your clocks!















Remember when PopSci did an article on an Aluminum-Air battery. If that had come to fruition then we would be filling up with aluminum chips.
“Don’t forget to water the plants while I’m away.”
“Yes dear.”
“And the battery.”
“Yes dear. The battery as well.”
1++
Air battery? *yawn!*
Joule thief? *yawn!*
Where’s the hack?
Did you not see the ugly ass clamp and the sharp edges ready to slice away at the unweary?
Smart ass comments? “yawn!”.
Saying “Where’s the hack?” yawn!
Benchoff trolling “yawn”
^ There he is! Who’s a good hack? Who’s a good hack?! Yes YOU are!
Is it really an aluminum air battery or just an aluminum copper battery with the standard two dissimilar metals?
My thought exactly. I used to have a calculator that “ran on salt water”. It had tiny little sponges you needed to soak in salt water, and those were pressed against aluminium and copper electrodes.
So in my opinion this would be an Al-Cu battery.
I wondered the same when I was making it. It still works if you replace the copper coin with a bit of aluminium. If I omit the carbon water filter and put the coin directly on the electrolyte-soaked paper, and clamp it, it produces only about 1/10th the current. Those two things make me think that it’s mostly an aluminium-air battery. I guess that another thing I could try is putting it in a CO2 filled container and see whether that stops it from working.
I don’t mean it wrong, but you can light a 40″ long floro tube using the earth battery which is related. I used a 350 volt Joule Thief to do this here: https://www.youtube.com/watch?v=5kL8ys8m0-4
Making a tube glow is easy – they sometimes glow like that from static electricity from your hand. Making it actually light up is not so easy.
The reason why there’s two prongs at either end is because there’s heater filaments, like regular incandecent lightbulbs, inside the tube. When the tube switches on, there’s a relay that lights the filaments up until the tube strikes through. You need the cathodes hot enough to cause the thermionic emission of electrons – otherwise the tube is operating in cold-cathode mode and its resistance is very high, and the efficiency very low.
When the cathodes heat up and thermionic emission starts, the number of free charge carriers increases dramatically and the current through the tube increases likewise, at which point the tube suddenly flashes into full brightness. The current running through the tube is then enough to keep the cathodes hot and the filaments are turned off.
This is also why fluorescent tubes aren’t dimmable – reducing the current or the duty cycle will make the emitters cold and the tube shuts down into a dim glow. If you use a battery to keep the filaments at both ends glowing just slightly, you can run smaller amounts of current through the tube and get it to glow at any brightness
Very cool! I might have to try that.
Thanks for the info!
Assuming you’re using some sort of boost converter to run the tube, one way to facilitate filament heating is to apply the AC output of the transformer to the prongs on the same side of the tube, and connect the opposite prongs to each other through a current limiter – a thermistor is typically used, but a regular resistor will do. An adjustable constant current regulator would be ideal for experimentation.
That way part of the input current is always diverted through the filaments and the tube can run at reduced power while still maintaining hot cathode operation. It’s a less efficient way to light a fluorescent tube but it effectively makes it dimmable.
It’s also possible to do clever tricks with the tube, like turning it into a rectifier, because heating one filament and not heating the other makes the tube into a diode – at least for small currents.
Would making the filaments slightly radioactive make it easier to facilitate breakdown?
I would assume so, but the beta emissions would themselves cause the tube to glow so you can never turn it off. The electrodes are already shaped and coated in a way that makes it easier to eject an electron into the tube.
A weak radioactive source can help ignite it by ionizing the gas in the tube, but you need the thermionic emission to provide the bulk of the current.
Also, modern fast start electronic ballasts don’t necessarily use the filaments at all – they just zap the tube with high voltage to strike it and then reduce the voltage for operation.
Glowing the filaments to start the tube evaporates the metal gradually and the gaseous metal deposits on the tube and on the electrode, interfering with the operation and eventually blackening the ends, so striking the tube directly makes it last longer.
The temperature of the cathode basically limits the amount of current going through the tube – no free electrons, no current.
http://www.belljar.net/fig6a.gif
Of course there’s some capacitive leakage at high frequencies and voltages which make the tube glow as well, but that’s a different mechanism and doesn’t really pick up at kHz frequencies – if you want to power a fluorescent tube by RF, you probably need to go into hundreds of kHz to MHz.
Dax: Well, that is the usual way to do it BUT, if you hit it with high voltage, in my case about 350 volts from the earth battery/JT/supercap combo, and a high frequency, in this case about 30,000 Hz, you can also get one to light up very well. What most folks do not get in many of my videos is that my cam is on auto exposure so, it closes down and makes many of my lights look not near as bright as they are in real life. I made a Pirate Light many years ago using this same high voltage/high freq. JT circuit and, on a single AA battery, there is way more than enough light to read by from many feet away. I use one when my lights go out in a storm. Now, I am certainly not claiming the lights are as bright when driven by a ballast at 110 volts and many amps but, for being able to get this much light, for free, from an earth battery’s humble output, I think it is not bad. I started with 1 led, then 3, then 100, then 200, then 300 then 400 leds lit from this same circuit before I started messing with these tubes. Most folks are lucky to get 1 or 2 leds lit from an earth battery. This was all about getting the maximum light from the minimum (free) input. Sort of energy harvesting on steroids. Thanks for your reply.
>” I made a Pirate Light many years ago using this same high voltage/high freq. JT circuit and, on a single AA battery, there is way more than enough light to read by from many feet away.”
I have done the same thing, and the tube is not running hot cathode. A single AA just can’t push the current to properly light up even the smallest 7 Watt CFL tube, and the battery cell just gets really hot. Basically, you’re throwing away batteries, as the same circuit with a LED would put out many hours of light instead.
I needed six D-cells to get the 7 Watt tube into hot cathode operation where it suddenly goes bright, but even that only works for so long because the current through the batteries is still over an Amp and alkalines simply aren’t built for that sort of drain. A good cell will do it, but probably not.
What you have to realize is that between hot/cold cathode operation, the efficiency of the tube changes a lot. When the tube is cold, most of the voltage drop happens near the ends just to get a free electron going through the tube, so most of the energy gets spent there and even as you’re pushing more and more current it doesn’t really turn into more light – it’s just wasted.
When the cathodes get hot enough, almost at the same current, suddenly the light output goes from a few lumens to hundreds of lumens, and for nearly the same energy input you’re not just reading a book at 3 feet – it lights up the entire room.
There’s no point in using fluorescent tubes in cold cathode mode because it’s so inefficient. As I said, if you’re using alkalines you’re just throwing away batteries.
I can run these circuits from “dead” batteries for hours. I have no idea why you think this does not work as I use these every day here in my small apt. My friends give me batteries that no longer work in their devices, and I can use them with this very same circuit and others, to produce bright light for many hours. I do not think you understand what is happening here, this work was done by me, and many others, about 10 years ago and we found that high freq. and high voltage can light both leds and floro tubes to get a lot of light for free. Then, I used this circuit on my earth battery and it works better than I ever expected. I run my Christmas lights (leds) every year from the earth battery for free. Give me one of your “dead” AA bats and I can use it to light a floro tube bright enough to read by all night long! These last for hours. I have one by my bedside that I stuck an old D cell into and, it has been over 2 years and I have still never needed to replace the battery. So, when you say it sucks batteries right down, that is incorrect. Quite the opposite. That is why I can get the earth battery to power them. If they needed a lot of mA’s, the earth battery would not be up to the task. Tesla did the same thing many, many years ago so this is nothing new. (That’s where we got the idea) The only sort of “new” part of this is the high voltage/high freq. JT circuit…that is only about 10 years old or so. I am not arguing with you here, I am just saying I have many of these devices running over here and unless you have built some, you might not get that there are other ways to do this more efficiently.
>”I have no idea why you think this does not work as I use these every day here in my small apt.”
Because I’ve built the same circuits from the same plans and found them lacking. My “dead” batteries have such a high internal resistance that they simply cannot provide enough current to properly light up a fluorescent tube or even the smallest CFL I can find in the stores – the voltage sags and the Joule Thief circuit cannot actually draw the battery empty.
Even a fresh battery only works for 15 minutes and then the tube dims down to a weak glimmer.
>”I have one by my bedside that I stuck an old D cell into and, it has been over 2 years and I have still never needed to replace the battery.”
Now I know you’re a crank with something to sell.
A D-cell has in theory 25 Watt-hours of energy at very low currents to dead empty. You need about 3 lumens of light to read, and a common LED gives you that with about 40 milliwatts. A full D-cell therefore gives you 625 hours of light – in theory – one hour a night for two years.
So you claim you’re running a very inefficient – in comparison to the LED – fluorescent tube from a Joule Thief circuit, from a spent battery, for two years. I call doube bullshit.
>”The only sort of “new” part of this is the high voltage/high freq. JT circuit…”
You claimed 30 kHz, I just checked my old notes about my experiment and found out I had 33 kHz. To push RF current through a fluorescent tube by force, barely above audio frequencies doesn’t cut it.
I am a crank with something to sell? Well Sir, you can go “Blank” yourself, how is that? I am selling nothing and these videos I made are back from about 8-10 years ago. (Please try to keep up) Now that you have launched the ad hominem attacks, that tells me you are confused and have run out of useful things to say. Fine, if you do not, or can’t understand this, no shame in it…just admit it. I have about 73 videos on youtube most of which deal with this and, wherever it was that you think you learned electronics, you need to go back and try again. Sheesh! Try looking up Big Clive, Tesla, Jose Pino, Jeanna, Faraday, Stubblefield, and Pirate Labs with a simple Google search. You might really learn something useful. I thought this site eliminated ignorant folks who troll but I guess they missed you. Here is a video from 8 years ago where I am lighting 200 leds from a single “dead” (under .7 volts) AA battery:https://www.youtube.com/watch?v=Co4WsKOcJk0
I guess you think I did not do this either? I have had “experts” tell me this can not work because each led requires 30 mA’s and that would mean 600 mA’s to light these and that “dead” battery can not do this so I must be faking it. Well, many folks around the world have replicated this, and many of my other videos so, just because you can’t seem to comprehend how this is done bothers me not at all. Good day Sir.
>”I guess you think I did not do this either? I have had “experts” tell me this can not work because each led requires 30 mA’s and that would mean 600 mA’s to light these ”
I’m not going to, because your fake argument has a gaping flaw. Thet 200 LEDs don’t need much more than a couple milliamps to “light up”, as in to provide a visible glow, but they need a certain amount of voltage to conduct in the first place, and voltage times current implies power.
Let’s take a red LED and give it 2.3 Volts of forward drop. Multiply that by 200 diodes and it becomes 460 Volts. Alright, then multiply that by let’s say 5 milliamps to make each LED in series put out a tiny bit of light. That becomes 2.3 Watts of power.
2.3 Watts of power out of an alkaline battery, even a full one at 1.6 Volts, would imply 1.4 Amperes of current. Problem is, a common alkaline battery has about 0.2 Ohms of series resistance so drawing that amount of current would cause the voltage to drop. In fact the maximum amount of power per cell you can expect – according to my experiments – is about 1.4 Watts per cell, and that’s only true for a fresh cell. A drained cell with a voltage below 1 Volts to begin with isn’t going to give you much at all.
What I’m saying is, you’re exaggerating your results, hugely.
I’m in no doubt that you can get LEDs to glow slightly from a single dead AA. I’ve made a red LED glow visibly by the current from a yellow LED exposed to sunlight and acting as a kind of a solar panel – but that’s just microamps of current.
What I’m doubting is that you can get hours and years of light from a magical joule thief circuit, from a fluorescent tube, because the efficiency of the circuit is obviously and by demonstration extremely poor and the energy left over in the cell is simply not enough to support your claims.
On the point of it, you’re promoting “More on Overunitydotcom in the Joule Thief topic. Also, see Infinite Power’s forum. ”
That’s just bullshit. You’re a crank – begone.
Infinity Powers Forum? What the hell are you talking about? I have never been there nor heard of them. Just because you do not want/can’t understand what is happening here, and it is not magic I assure you, then there is no reason to make fun or try to claim my results are fake. I suppose then you are calling all of my replicators fakes too? They will be happy to hear that I am sure. Leds do NOT need power to light…period! High voltage/high frequency does the trick at a very low, low mA draw. This has been proven, not just by me, but by many others a long long time ago so you are arguing about something that has been done, and IS being done, many times now. I am a Moderator over at Overunity.com and I have no idea what your problem is with Stefan Hartman’s site. Possibly it is because you do not understand the term overunity? That would be my guess. IF you did, you would know it does not mean any 2nd law of thermodynamics violations…not at all…but you would have to have actually done some research to learn that. You just seem to want to pretend that you know everything and sit over there and tell me my lights, that are running right now as I type this, can not be running. Maybe one day you might realize how stupid that sounds? My electric bill over here averages $30/month and that is heat, air, hot water electric range…fridge..everything. My guess is that yours is a lot higher perhaps? I guess you did not see my video where I use the same circuit to light a Cree 800 lumen mains led bulb with a “dead” AA battery? Suppose not. You just don’t want to do the work…you are lazy.
I am done with you as you have already proven your ignorance beyond any doubt so, until you learn to do some research, you will remain so. You are so scared and backed into a corner you have to make up stuff I never said, like that infinity forum B.S. you made up .very sad dude…get a life.
[Pirate Labs], your YouTube link literally reads “More on Overunitydotcom in the Joule Thief topic. Also, see Infinite Power’s forum.
Thanks.”, in the description for the video by [Pirate Labs]…
Fun read :) If only they were used more often.
Most excellent (good ones, not the cheapos) flashlights seem to switch into ‘joule-thief’ mode when the cells are drained enough.
*flicker flicker flicker*
…but, they’ll drain each AA down to about .4 volts. (Not scientifically confirmed by me.)
Almost every last possible drop.
notarealemail: There was no reply button after your comment so, I hope you see this here. Yes, you are correct and I apologize for not remembering/knowing who infinite power was. (I still don’t even after a google search.) the only thing I can think of is this was 9 years ago and sometimes folks would send me components or parts and I would recommend their site. It does not seem to exist anymore so I removed it from the video description. So, Dax, and you were correct about that and I was wrong. I have no idea who that was.
Okay.
I’m tempted the pry-apart some old film cameras to test out just what it can do anyways.
I know the modifications, remove giant cap and one of the diodes.
Rainy-day for fun project.