Graphene has attracted enormous interest for electrically detecting chemical and biological materials. However, because the super material doesn’t act like a normal semiconductor, transistors require multiple layers of the material, and that’s bad for 1/f noise especially when the transistors operate at maximum transconductance. Researchers have found a way to operate graphene transistors at a neutral point, significantly reducing 1/f noise while not impacting the sensor’s response.
The team created a proof-of concept sensor that could detect an HIV-related DNA hybridization. The sensor was able to detect very tiny concentrations of the material.
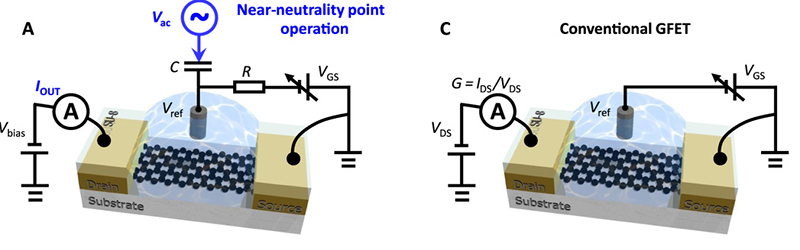
The ion-sensitive field-effect transistors (ISFETs) employed in sensors operate at maximum transconductance — the current gain attributable to the input voltage. That makes sense because a small change in gate voltage caused by material creates the largest change in current.
However, earlier research suggested that the noise spectrum of these ISFETs are lowest near the neutrality point of graphene. Using large magnets can achieve this neutral bias, but that’s not very portable or practical for sensors. The new research shows a way to apply a sine wave to the gate of the transistor to cycle it around the neutrality point and then monitor the current in a common-source configuration.
Coincidentally, the team used a lock in amplifier, which we recently talked about. We’ve covered lots of ways to make graphene and its uses seem to be unlimited. We think there are a lot of opportunities to blaze new trails with this new material for the fairly equipped hacker.















Can someone point me to why several layers worsen 1/f noise?
Possible explanation: 1/f noise is a surface effect, multiple layers have a larger surface.
(maybe I’m wrong)
It increases the planes in which thermal electrons can move.
Aren’t multiple layers of graphene called “graphite”?
Perhaps but with each layer engineered for a specific purpose and fitted together correctly aligned to place the right parts of each in contact with one another. This isn’t your typical plain old chunk of graphite that acts as a brush in your drill motor or that you write with in your pencil.
Graphite is amorphous carbon, Diamond is carbon mono-crystal, graphene is a different structure of carbon.
No, in the same way stack of papers isn’t mdf.
Yes, per wikipedia graphite is layers of graphene when graphite is in the crystaline form and I am not aware of graphite being amorphous either until I read there are forms that are amorphous. I thought that was one of the forms of coal that are amorphous and I guess they are considered graphite. What, is there a ligand or glue or something in between or dope like coal or something carbon transistor else? Read the Wikipedia entries for Graphene, Graphite and Coal. They help to clarify for starters.
Interesting exercise in how understanding the problem adequately makes it easier to come up with a solution.