Rechargable batteries are great – they save money and hassle when using portable devices. It’s pretty common to want to recharge a battery, but less common to intentionally discharge one. Regardless, [Pawel Spychalski] is working on a device to do just that – in a controlled fashion, of course.
[Pawel] himself notes that the device isn’t something the average person would necessarily need, but it does have its applications. There are times when working with various battery chemistries that it is desired to have them held at a certain state of charge. Also, such devices can be used to measure the capacity of batteries by timing how long they take to discharge when placed under a given load.
The build is one that takes advantage of the available parts of the modern hacker’s junkbox. An Arduino is used with an N-channel MOSFET to switch a resistive load. That load consists of load resistors designed for automotive use, to allow cars originally designed for filament bulbs to use LED indicator lights without the flash frequency speeding up. The resistors are 10 ohms and rated at 50 W, so they’re just about right for ganging up to discharge small LiPo batteries in a short period of time.
[Pawel] has tested the basic concept, and has things working. Next on the agenda is to find a way to get rid of the excess heat, as the current design has the resistors reaching temperatures of 158 °F (70 °C) in just a few minutes. Use some of that power to drive a fan?
Perhaps you’re working with lead acid batteries, though – in which chase, you might want to consider blasting away the sulphates?

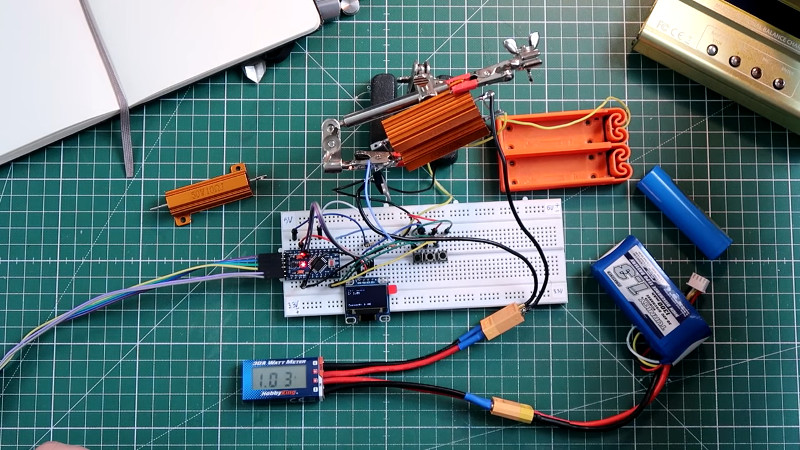














Those power resistors need to be bolted to a heat sink.
They are rated 50W when bolted 12” x 12” x .059” Aluminum panel, as per the data sheet.
In the intended automotive application at 13.8V the resistor is burning 19W, and only intermittently when ever the turn signal is on (50% duty cycle would only be 8W), which is why they can be used without a heat sink.
Yeah, but it seems tough to damage wirewound resistors. Sometimes I use these at ~150% of their rating to make cheap heating elements; the solder around the lugs tends to melt, and so does the adhesive they use to stick the resistors in the heatsinks that they ship in, but the resistors tend to work fine.
I’ve also used them as heating elements, but I did once manage to blow one up. The chunks of filler material flew out on either side and covered my desk. I did however significantly overload them though, as I was getting impatient waiting for them to reach temperature…
RC airplane types could use a discharger. Storing lithium batteries at full charge will make them start to puff up in a season or two, so you should discharge them to ~50% or so before storing them.
Because of this, most RC chargers have a built in discharger.
In fact, most RC chargers charge and discharge several types of lithium cells and also nicads and lead-acid cells and prices start at around $40.00. At your hobby shop or online.
That’s actually the whole reason this was built. The author’s charger could only discharge at 400mA. Not fast enough to be practical for regular use.
70C is perfectly fine for a resistor as long as you don’t touch it :) They’re probably going to be at 150C or so internally at their rated power, installed on their intended heatsink.
Without that heatsink, obviously you must derate significantly.
this build is neat but way overthought. most lipo chargers have a discharge on them as previously stated. Also if you want to discharge something you do not need to over design something like this to do it. I needed to pull down a few cells recently in a pack it was much easierr to use a fan frrom a pc and leave my meter connected to it rather than this whole contraption.
Not quite as couth, but I use a high power headlight (12v) bulb. Yep, resistance varies with voltage, but with a coulomb counting meter, it doesn’t matter.
Perhaps you’re working with lead acid batteries, though-
“Desulfation” isn’t a thing outside normal operation. Yes when you charge a lead acid battery you convert lead sulphate into lead oxide/lead and hydrogen/sulfuric acid. The reason your car battery now sucks is because the lead sulfate slowly pushed the lead oxide and lead powder off of the current carrying electrodes, slowly destroying the plates.
It’s a mechanical failure not a chemical one. There is no way to put them back together to recover the current capacity or voltage without complete reassembly. Ie battery sulphate blasting is bullshit.
You can get 150watt 20amp constant current electronic loads on eBay for under $40. I use one to test power supplies.
An interesting variant on this is charge another battery, rather then create heat. So your half used batteries can be combine for one last flight.
I judge the local high school and jr. high science fairs. There are always kids who do battery comparison projects. Brand X vs. Brand Y. Alkaline vs. Carbon vs. NiCad vs. Lithium, etc. At this years competition, I will be sure to mention this article to some of the kids.
I could have done that with a 555.
(Well, a 555 could discharge a battery if it is hooked up to one…)
B^)