If an object is conductive or has been given a conductive coating, it can be given a metal skin via electroplating. Electroplating is a simple process that is perfectly accessible to anyone in possession of vinegar, salt, a power supply, and some metal such as copper or nickel.
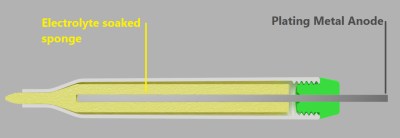
The process might be simple, but as with all such things there are a few gotchas. One of them is this: because electricity follows the path of least resistance, recessed areas of an object may not electroplate well (or at all) no matter how long the object is left immersed. To address this, [Brodie Fairhall] designed a 3D printed electroplating marker. The marker is essentially a more refined version of brush plating, and allows more precision and control than full immersion in an electrolyte bath.
[Brodie] created an excellent video that explains all one needs to start electroplating, and demonstrates using his marker to electroplate complex recessed shapes. Watch him coat a 3D-printed cat pendant in both copper and nickel in the video embedded below. It’s concise, well-edited, and chock full of useful tips.
Electroplating isn’t just for 3D prints or small metal objects. It can also let you plate your own PCB vias from the comfort of your own workbench.
















This reminds of the Selectrons process used to build up wear on shafts and bearings on industrial equipment circa 1984, exploited natural selection of wear regions by constant application pressure and motion to ensure only the best adhesion occured. Finishing off with lathe still necessary on rotatable parts.
The approach here could be used for a range of nifty surface sensor prototyping on PCBs, thanks for post :-)
Your post didn’t make a lot of sense.
Basically you’re referring to http://www.selectron-oberflaechentechnik.de/description.htm, who use electroplating to resize components that are out of dimensional tolerance due to wear or corrosion.
Didn’t make sense? You just restated what Mike said (arguably with better grammar, but shorter on actual info).
This video is dangerous. NEVER use salt (NaCl) as an electrolyte. Electrolysis will split off Cl ions and they will form chlorine gas which can be very dangerous to inhale and is poisonous. The gas will also attack surfaces and objects in your shop/garage too.
Hackaday should not be promoting such videos which may harm people who try this method.
Most electrolytes for plating are quite dangerous in some way or another (strong acids, poisons etc) this method produces significantly less Chlorine gas than electrolysis of brine (salt water) it also produces small amounts of Hydrogen.
I’ll add a note the video comment telling people to do this in a well ventilated space.
Hm. So you’re saying that adding salt to an aqueous solution will produce less chlorine gas than salt water. Seems unlikely.
Use Epsonsalt instead. It’s saefer.
Almost all electrolytes for plating are dangerous, the current levels used in this process are very low and as a result the volume of gas (both Chlorine and Hydrogen) produced is very low.
Plating should be done in a well ventilated area.
This process can be done without the salt, but it will be significantly slower or other electrolytes can be used instead.
Does anyone know a rough figure for how much chlorine gas would be produced for an average use case like this electroplating application?
The quantities are minute, you probably get exposed to more chlorine from walking past a swimming pool, or running a bath if your water is treated with it.
Engineer here. I use electrochemical etching for steel components at work on a daily basis. While I really don’t think that NaCl can be effectively used efficiently for electroplating (usually an ionic salt containing whatever metal is going to be deposited), I disagree that the chlorine gas production is as dangerous as you make it out to be.
I believe that chlorine gas production in a voltaic or electrolytic cell depends on the potential (voltage) applied across the electrodes.
Basically there are two ways these electrochemical reactions can be driven:
1) a lower potential reaction (lower voltage) results in electrochemical etching or electroplating
&
2) a higher potential reaction (higher voltage) results in chlorine gas production
Another way to think about it is that NaCl is a very, very stable ionic salt. It takes a certain threshold to remove either ion from the system especially when there is another possible reaction that is ‘preferable’ (I don’t have time to go into this).
Electroplating is an easy process, chemically. Ideally, the only changes to the system is the build up of metal on one electrode and a removal of metal on the other. The plating solution should retain the same number of ions.
However, to produce chlorine gas (much more complex) there need to be enough electrons to balance out breaking H2O & NaCl into H2(g), O2(g), NaOH(aq), & Cl2(g).
Of course I mean *produce chlorine gas in larger quantities.
interesting, any ballpark figures on which voltage would be a safe margin?
Electrochemistry is all extremely low voltage. Look at standard reduction potential voltages on a graph: the maximum useful voltage is maybe 3V. More than that and all you’re doing is wasting power. If you have a situation where the reduction potential of your target metal is less than the oxidation potential for chloride (-1.36V) you shouldn’t produce chlorine gas. But that’s unlikely to be the case unless you’re I dunno lead-plating something.
I think the ideal here is to have a voltage high enough to get good plating (considering the resistance of your electrolyte, so you’re not limiting your current, which is all that really matters) but no higher, and at that point you should evolve roughly as many chlorine atoms as you plate on metal ions (that become atoms.) (This is more complex than it sounds: if you’re plating on something that takes three electrons to reduce, like aluminum, you’ll end up dumping out three chlorine atoms. But it’s within an order of magnitude.) As your voltage increases past that you start electrolyzing the water and producing hydrogen and chlorine, so then you’re producing more chlorine than necessary.
(Note: it’s been 20 years since I’ve taken electrochemistry, so I’m doing this by memory. This is not at all what I do for a living, so I may have some minor or gross errors in this.)
With all that said, I’ve made sodium by electroreduction, which I know for sure was producing chlorine, and I know what chlorine smells like, and in making 1mm balls of sodium I could not smell the amount of chlorine I was producing. It did rust metal immediately adjacent to my setup. If you’re really worried you could build a fume extractor that blew the evolved gas through a breathing filter for chlorine (they’re cheap) or drew it across powdered iron to absorb the chlorine.
thanks
There should be a rule on the Internet that people who say “X is dangerous” should have to quantify the danger and suggest ways that it can be mitigated.
Maybe an MSDS-level description is asking too much, but at a step in that direction?
For instance, the surface area plated here with a pen looks to be a square inch or so at the most. How much chlorine gas can you produce? Is it sufficient to open a window, or must the entire neighborhood evacuate?
In short, “X is dangerous” is almost always correct, but it’s also entirely vacuuous. Water is dangerous, never mind a drill press. It’s all about knowing the danger and taking the proper steps to mitigate it.
Hmm, just had an idea for new type of metal 3d printer… https://reprap.org/forum/read.php?1,185062,185278 ehh, too late :)
Not just salt as dangerous solution – a lot of plating solutions are caustic, contain cyanides, etc. I last did electroplating as a middle school science fair project — using solutions from a company that was a client of my father’s — and most couldn’t be brought into the science fair facilities. I’d be very interested to hear what sorts of plating solutions are available commercially or with easy/safe recipes.
Great video – the pens are available commercially, but they’re stinkin’ expensive. I’d love to see him do one on anodizing aluminum.
Now somebody that already has their 3D printer modified to use as a plotter…
But this reminds me: in a Tektronix video from the 1960s, I remember seeing that the way they etched their PCBs was to selectively gold plate copper PCBs, where they want traces to remain (they used a photoresist in the selective plating process). The gold acts as an etch resist. I’m tempted to try doing this with “liquid tin”, the electroless tin plating solution used to plate PCBs, but using a “plating pen” like this with other metals such as nickel seem more likely to work.
Why would you want do do it this way, when you can just directly etch the copper? Because this gives you a solid copper plane to facilitate plating of the vias. The order of operations is, drill, plate the holes, photoresist, gold plate, etch. The gold plating protects the vias as well as the traces from etching.
This looks fun and makes me wonder about clothing apps too somehow. The etching idea above gets me thinking about gold plating boards traces post etching or post repair(s) or upgrade(s) too. Neat
This looks fun and makes me wonder about clothing apps too somehow. The etching idea.industrielle galvanische behandlungen