Everyone one of us is likely aware of what lead — as in the metal — is. Having a somewhat dull, metallic gray appearance, it occupies atomic number 82 in the periodic table and is among the most dense materials known to humankind. Lead’s low melting point and malleability even when at room temperature has made it a popular metal since humans first began to melt it out of ore in the Near East at around 7,000 BC in the Neolithic period.
Although lead’s toxicity to humans has been known since at least the 2nd century BC and was acknowledged as a public health hazard in the late 19th century, the use of lead skyrocketed in the first half of the 20th century. Lead saw use as a gasoline additive beginning in the 1920s, and the US didn’t abolish lead-based paint until 1978, nearly 70 years after France, Belgium and Austria banned it.
With the rise of consumer electronics, the use of lead-based solder became ever more a part of daily life during the second part of the 20th century, until an increase in regulations aimed at reducing lead in the environment. This came along with the World Health Organization’s fairly recent acknowledgment that there is truly no safe limit for lead in the human body.
In this article I’ll examine the question of why we are still using lead, and if we truly must, then how we can use this metal in the safest way possible.
The Miracle Metal

An interesting detail about lead is that it doesn’t just have one stable isotope, but that it has a total of four stable forms, with three of these being the most common. These are 206Pb, 207Pb, and 208Pb, which result from the nuclear decay of 238U, 235U and 232Th respectively. 204Pb is the primordial form, meaning that it was created along with the other materials that ended up forming this planet and does not result from any decay chain. This makes 204Pb useful for radio dating rocks by comparing its ratio with the other (radiogenic origin) stable lead isotopes.
Lead is easily obtained from galena (lead(II) sulfide), requiring only relatively low temperatures to extract the lead. The use of lead as an everyday metal developed throughout the prehistoric into the classical era, with the Ancient Egyptians being the first to use it in applications like cosmetics, a practice that would later spread to Ancient Greece and into post-medieval Western societies. The Ancient Romans would use lead extensively for water pipes (the English word ‘plumbing’ being derived from the Latin word for lead (plumbum), where its resistance to corrosion was highly praised.

Lead saw significant use as a writing material, in the form of lead tablets, as well as for slingshot bullets. It was also part of the wine and food preparation process, as sweeteners and preservatives that were prepared in a lead(-lined) vessel would impart a pleasant sweet taste to the food and wine on account of the lead(II) acetate (‘sugar of lead’) that was formed, compared to the bitter verdigris one would get with copper or bronze vessels.
Starting in the 11th century, European architecture used lead extensively for roofing and piping, also evolving into its use in stained glass. The invention of the printing press meant a key role for lead, as did the invention of (practical) firearms. Lead was an essential component in whitening cosmetic (Venetian ceruse), which were heavily used by Western European aristocracy and royalty, as well as by Japanese geishas starting in the 18th century.
Lead Exposure Routes
Europe’s Industrial Revolution saw lead production for the first time exceed that of Ancient Rome, with lead being extensively used for plumbing, paints and lead-acid batteries. This was also the era when physicians began to strongly link exposure to lead with a variety of mental and physical disorders associated with ingesting lead. The UK would enact its first laws aimed at reducing lead exposure in factories in the 1870s.
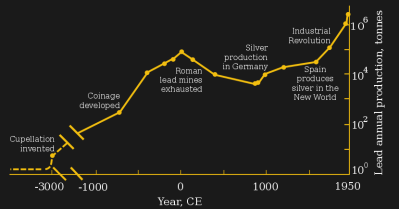
By this time it was primarily occupational exposure to lead that formed the main risk: people mining lead ore and those involved in smelting and otherwise processing the metal would breathe in the lead dust and vapors, and ingest lead (by licking one’s lips, etc.). Absorption through the skin is less common, but as in the case of lead-based cosmetics can happen in the form of long-term exposure to low (but constant) amounts of elemental lead.
The frustrating thing is that humanity had previously discovered and seemingly forgotten the dangers of lead exposure. During the 2nd and 1st century BC Ancient Greek and Roman scientists and physicians noted the effects of lead on the human body, describing symptoms such as gout, mental degradation, colic and paralysis. While the Middle Ages saw a general ignorance of the dangers of lead, the centuries after it saw a sharp rise in the awareness of the dangers of lead exposure.
Lead is a Dangerous Mimic of Calcium
Lead dust can easily enter the bloodstream, with nearly half of the lead dust one breathes ending up in the lungs, of which almost all gets absorbed into the blood where it binds to erythrocytic and plasmatic proteins. From there it is distributed to the remaining tissues of the body. At this point the cellular mechanisms that underlie lead’s neurotoxicity and general toxicity take hold. The half-life of lead in the blood is about 35 days, while it can stay for 2 years in the brain and many years in the bones, providing a long-term reservoir that becomes especially risky in case of extreme long-term physical exertion, such as a pregnancy.
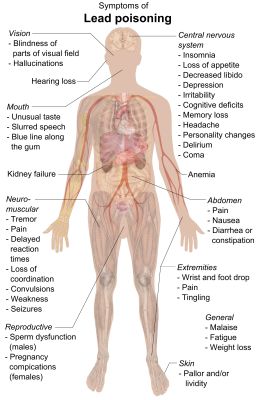
The main risk in lead exposure lies in how it interacts with the body’s biochemical processes. Because of its chemical composition, it can take the place of calcium in the body, as well as that of zinc and magnesium. Because of this it gets easily incorporated into virtually every tissue of the body, where its mismatch with an actual calcium, zinc or magnesium molecule proceeds to cause enzymes to fail and stop functioning. Worse is that lead has a larger ionic radius than zinc and calcium, meaning that it more readily binds to enzymes than the latter two.
After binding to an enzyme, this biochemical disruption can impair a wide variety of processes. The most dangerous of this is probably lead’s interference with the delta-aminolevulinic acid dehydratase (ALAD) enzyme, which is crucial for the biosynthesis of heme, the cofactor in hemoglobin. If lead levels are high enough, this leads to anemia, as well as damage to neurons due to build-up of aminolevulinic acid. In addition, lead causes DNA damage and destroys a cell’s structures because of disruption to critical cell processes.
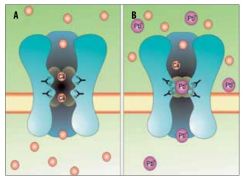
Because of this same mimicry of calcium, lead is able to easily pass through the blood-brain barrier via the calcium-ATPase pumps of the cells that form the barrier, the same way lead easily enters other cells. Here too, its biochemical differences with calcium causes it to disrupt the biochemical processes it interacts with, causing disruption of synapse formation, neurochemical (neurotransmitter) development, loss of ion channels, and generally stunts the development and overall functioning of the brain.
Lead ions (Pb2+) function as NMDA receptor blockers, being biochemically similar to Ca2+ ions, but end up blocking the receptor’s channel pore, effectively disabling it. Unlike Mg2+ ions, the Pb2+ions aren’t easily dislodged. Depending on the amount of lead ions in the brain, the resulting effect can be mild to crippling. Combined with the other neurotoxic and cytotoxic effects of lead, severe brain damage is to be expected.
Lead in the 21st Century
Although lead-based paint and lead in gasoline are mostly a thing of the past now, environmental contamination of lead is still very much present. As a heavy metal, it doesn’t decay, meaning that the lead pollution from industrial and other processes — such as energy production from coal — is still around, with lead contamination especially in cities is quite profound. Any exposed lead will end up in the environment, via (rain) water or as dust, making it an ongoing issue.

Like humans, plants readily absorb the lead in the environment, where it can end up in our food and that of other animals whom we consume later. The inhalation of radioactive 210Pb (and subsequently 210Po through decay) as a component of cigarette smoke from these components which the tobacco plant absorbed while it was growing is still a major issue, although here the main problem is mostly caused through these alpha particles and not the toxicity of the lead itself.
In civilized nations at least, workplace safety has improved to the point where occupational exposure is minimal, and significant lead exposure for the average person is unlikely. Contributing to this has been the work of countless researchers during the 20th century who have made the case for lead toxicity, resulting in the World Health Organization (WHO) along with the US CDC reducing the ‘acceptable’ limit from about 50 µg/dL in the 1980s to <5 µg/dL today, with no safe limit.
Efforts are underway to ban lead as a component for bullets, as these cause lead contamination of the environment. Any lead that remains in an animal after it has been shot can end up being consumed by humans as well as predators and the like. Alternatives for lead are steel, tungsten-nickel-iron, bismuth-tin, and tungsten-polymer.
Lead at Home
Barring existing contamination such as leaded paint in the US, lead contamination at home is often self-introduced, such as when handling lead in some form during construction work, creating or handling lead fish sinkers, lead bullets and similar (including casting). The use of lead-containing solder alloy is also still a common practice and likely touches each person reading this article. For all of these scenarios the same rules apply:
- Do not eat or drink at the working area.
- Do not put lead (or lead solder) in your mouth.
- Do not breathe in the fumes.
- Do not touch your face while handling lead.
- Clean your hands with soap after handling lead.
- Wet clean any surfaces that may be contaminated with lead dust
This is especially crucial at home where children and pregnant women are more likely to be present, due to the disastrous effect that lead’s neurotoxicity has on the developing brain and other tissues of a child and fetus.
Times Change
Back in 1983, the US Navy commissioned a study (PDF) to examine just how pervasive lead contamination was in an environment where lead-based solder was being used for soldering work. Because of the generous WHO limits back then, it was considered not a real issue assuming one took the above precautions into account. When compared against today’s WHO recommendations, the same precautions still apply, but 36 years later the recorded amounts of lead dust would be significant cause for concern.
One thing about heavy metals is that, like radiation, you cannot see it even when it’s there. The lead dust flaking off and coating surfaces, equipment and your own hands is invisible, and unless it’s in a form your tongue can detect (like lead(II) acetate), you’re unlikely to notice that you just ingested it. Nor will you taste the lead in vegetables, rice, or that piece of venison. The effects of lead-poisoning can take years to become apparent, by which time it’ll be too late.
The advantage we have today is that after thousands of years of experience with this heavy metal, thousands of observations and courtesy of modern sciences a good understanding of why exactly it is that lead is so dangerous to all life on this planet. Because of this, we can still keep making use of its blessings while taking further measures to prevent it from harming us and the environment.
















I was in Japan last October, and while in Kyoto, I asked the Geisha for the history of the white makeup.
She indicated “Back in the day, with candles for lights, the low lighting on a stage made normal skin difficult to see”.
So they chose the white makeup.
Today, it’s grandfathered in I guess.
There is also a cultural issue. Across the whole world, men have slightly darker complexions than women. (The reason why is not entirely clear but probably has to do with women needing to synthesize more vitamin D.) As a result, lighter complexions are considered more feminine.
Early on I’m sure the women that stayed at home to raise the family, and the man out in the fields, may have had some small impact on skin coloration. Today, those gals are all about the tanning salon, and surfer tans.
The much more significant cultural issue is that traditionally (and still today in much of the world), most people had to work outside, and got tanned dark skin. Only the really rich didn’t need to work outside and so could have lighter skin. So light skin in most cultures is seen as a desirable trait, suggestive of wealth and status.
More recently in the west, with naturally light skin, and crappy sun, we’ve started to see tanned skin as desirable as it suggests exotic holidays, suggestive of wealth and status.
Women in China go to extremes to avoid sunlight, including tent-like beach wear. The whiter the better – phone apps for this are popular. It sounds silly, go figure.
Also, on lighter skin it is easier to see facial features by light and shadow. There must be some kind of sexual selection involved.
So, they ended up looking like Michael Jackson…
(sigh!)
MJ definitely looked better white than he would’ve with Vitiligo and 2nd/3rd degree burns. The real problem was all the other surgeries (not all of which were cosmetic – his first nose job was for a broken nose and the second for breathing trouble after the first job).
https://youtu.be/afwO_MZjRjA
Lead is so nasty! I’m glad that lead free solder and batteries are replacing leaded.
About the only consumer application with no replacement I know of is PZT piezo ceramic.
The most common lead battery is the car battery, and that is not being replaced with a lead-free version any time soon.
If even a few of those get tossed into streams, that’s a lot of lead.
Hybrids and EVs use NiMH or some sort of lithium battery nowadays. If there’s a lead acid aux battery, it’s a much smaller one.
lead acid batteries have a good recycling industry going. any time where they contaminate the environment is when people irresponsibly dispose of their spent batteries. most local dumps have a free hazmat day for that kind of thing. last thing you want to do is get rid of a highly recyclable technology like this and fine the living shit out of the idiots who just chuck them into the ocean.
Scrap yards will pay you for used lead acid batteries. There’s money in them there batteries!
Motorcycle batteries tend to switch to lithium batteries nowadays, though this is mainly for weight considerations. I don’t know if cars will follow the trend.
I don’t know, EVs are getting popular pretty fast. They might not be practical now, but neither were LED bulbs.
Besides, car batteries are AFAIK one of the most recycled things on the planet.
Electronics are “recycled” in ways like burning in a heap somewhere out of sight, dumped in landfills, or occasionally hacked by DIYers with hand soldering irons, complete with little tiny bits of solder all over their workbench that probably fell off a sponge.
you can have my leaded solder when you pry it out of my cold dead hands.
That reminds me of the other day when someone on my team used my gear, and thought my iron must be really crap because they kept getting cold joints.
None of them were actually bad joints in any way I could see, but they sure looked like it if you’re used to lead!
Your leaded solder is a pretty small amount and a likely fairly low risk to anyone aside from maybe you, but I’ll stick to my lead free stuff and a wide chisel tip. It’s not particularly hard to solder with a good iron.
And I’d rather the companies that make iCraps by the millions for everyone to later flush down the iPot use lead free. No matter how well you keep the solder itself from polluting, mining that stuff by the ton is still an issue.
I though that lead solder fumes didn’t contain lead though? You still shouldn’t breath in the fumes though that’s for different reasons than the article implies.
If you look for example at the US Navy study, you can see that there is lead vapor in the fumes during lead-based soldering, even if the amounts are relatively small. It’s not nearly as much as with smelting and casting lead, obviously, due to the lower temperatures, but it’s not zero.
It is also, fortunately, easy to control at the source. If you use or work on gear that has, lead based solder, fume extraction (with proper filtering) is a must. It is not easy to get enough exposure through hand soldering as a hobbyist, but it is possible, and proper precaution should be used.
The low vapour pressure of lead at the temperatures in question, and the rapid cooling in proximity to the flux fumes, makes me wonder how much of the lead is metallic vapour of particle, how much is simple lead compounds, and how much is condensed on flux fume.
This is one of the many things I deal with at work where I need to know enough for the job to be done safely, and that is in large part knowing when to say “tag, not it!” (I’ve been through a lot of training, but handling lead, or other things like cadmium coatings, is not my job.) Job on a government vessel a few years ago got hung up for lead abatement. Site managers didn’t want to do it. We weren’t going in to do weld prep and welding without cleaning and certification. Their guy pulled an air sample and surface wipe that was clean. Our guy uncapped a lead test stick and whacked the plating with a drill hammer raising dust. Stick changed color. Abatement was done for the work area.
Lead-tin is an eutectic mixture that melts well below the melting point of the individual metals, which is why it doesn’t emit so much lead vapors as you’d imagine – the lead in isolation would still be solid – it has very little vapor pressure.
The smoke that comes off is the flux smoking, and any lead particles dissolved or caught in the flux.
Actually, if you look at the results of that study, you will find there was no elevated lead found in any of the workers after soldering. There has been another study done on this with factory (!) workers that are around wave soldering machines and such (where there is much more lead going about), with the same results.
Lead doesn’t really form fumes until about 450 degrees C and higher, which are, hopefully, temperatures nobody solders at. So you won’t get lead poisoning from breathing the fumes.
That said, the fumes are not good for you for different reasons – 99.9% of the fumes are created by burning fluxes and insulation of the wires you are soldering. That is both toxic but also irritating, exposing you to a risk of asthma and similar issues.
It’s all about the partial pressures. At lower temperatures the partial pressure of lead during a phase transition (solid to liquid in a eutectic mixture) is very low. At higher temperatures the partial pressure increases slowly until the boiling point of lead at which point it skyrockets.
And yes, almost all of the smoke from solder is from the resin used as a flux. That’s another topic.
Jan, Thanks for qualifying the comment about lead particles in fumes from soldering. I honestly don’t know why people still believe that they would exist in the fumes from standard electrical soldering practices when lead vaporizes around 1,100F.
In the study they did find lead in some of the air samples. You’re forgetting about lead dust, which forms any time the material is stressed (uncoiling a roll of leaded solder, for example). Most issues with occupational exposure to lead are about lead dust and not lead vapor, after all.
This makes a lot of sense. If i straighten a bit of solder that has been wrinkeled/bent, my fingers will have a grey stripe on them. Of course i immediately wash it off, but i think it’s the biggest way in which i am exposed to lead.
Luckily it will have a hard time penetrating the skin in the short time it’s on there.
As well as particles being picked up from the molten pool, both metallic and in compounds or complexes, by the flux fume, and particles expelled from the molten pool
You solder something at those temps it’ll end up scrap so lead is not really a major constitute to worry about now the flux flumes on the other hand.
Are there lead-free solders that work just as well for everyday hobby soldering?
I have both lead-based and pure tin solder. The lead-based solder is certainly easier, but the pure tin solder does work just fine with a bit of flux and a slightly hotter tip.
Except when you then take your work outside to below room-temperatures (<13 C), where the 99% tin alloys usually sold to hobbyists slowly start to form tin pest and disappear into gray dust.
This is especially insidious with RoHS tins used on boards like Arduinos, because the gray tin is not conductive, but it returns back to tin at room temperature, so you get intermittent failures that magically fix themselves when you try to diagnose the problem.
I’ve come to like Felder EL solder (“Sn100Ni+”: Sn99,25Cu0,7Ni0,05(AgGe)) rated for -96°C to +150°C. Melting point is 227°C.
It’s a long way from Sn99.3Cu0.7 to modern lead-free alloys though. Too bad that hobbyist use of alloys that actually work is discouraged by the high price.
https://www.researchgate.net/publication/42796230_Tin_pest_in_Sn-05_wt_Cu_lead-free_solder
Does adding trace amount of nickel, silver and germanium really get rid of tin pest?
Just because the solder is rated for low temperature does not mean it won’t develop tin pest – it just means it retains its mechanical properties instead of becoming brittle.
I wouldn’t use pure tin solder for anything.
No.
To someone who has never known any better they work well enough.
They do not work as well as good old 60/40.
Or maybe I’m just looking at it wrong. I’ll try to be more positive.
– Lead free solder requires higher heat increasing the likelihood of damaging the part or leaving burn marks on the PCB. With lead free solder you can master electronics and pyrography at the same time!
– A well soldered connection made with lead free solder is so dull that it looks just like a really bad cold solder joint created with leaded solder. Seriously, want to hide your 30 years experience with a soldering iron and make work which looks like that of a beginner? Switch to lead free solder.
– Just to function at all lead free solders require much harsher chemicals in it’s rosin core. It’s smoke is therefore more toxic than even the leaded variety only in a different way. Do you prefer lung cancer over losing a couple of neurons that you will never notice? Just ditch the lead.
– Lead free solder joints tend to form tin whiskers reducing the reliability and limiting the operating life of whatever you build. They aren’t even allowed in critical medical or space going devices for this reason. Lead free is a great choice for fueling the throw-away society. Go consumerism!
There are – the better question is where to find them and at what price?
The usual suspects like hardware stores only sell 99% tin, or tin-copper alloys that are really meant for joining plumbing bits and require over 300 C temperatures to melt. Having to order the right stuff online from abroad is a PITA because of the lead times and shipping costs.
For general purpose use, Sn96 is the best I’ve come up with. Trouble is, the outlets that sell solders don’t themselves know what they’re selling and don’t bother looking it up. They may say it’s Sn96 (Sn96.5Ag3.5) or SAC305 (Sn96.5Ag3.0Cu0.5) but then you get a composition like “Sn96.53Cu0.7Ag0.3”. Eh, close enough, we’ll say it’s Sn96.
But what is that stuff? What are the properties? The numbers don’t even add up to 100%. Who knows – I can’t even find it by google, and the closest stuff in composition is actually SN99 Ag0.3 Cu0.7 so it’s very likely they’ve just mis-labeled it.
https://nepp.nasa.gov/whisker/reference/tech_papers/2011-kostic-Pb-free.pdf
Pages 61 – 63. The solder should have at least 0.5% of antimony or bismuth, or more than 5% lead in it to prevent failure from cold temperatures. Silver and copper make matters worse. If you’re doing any soldering in a car that sits outside in the cold, or equipment that’s supposed to handle freezing temperatures, almost all of the lead-free solders are a bad choice – you’re relying on the equipment getting warmed up above 13 C before the tin pest starts to take hold.
The true answer is probably no.
At least I don’t know of any. 63/37 Lead tin is pretty hard to beat.
That doesn’t mean that there aren’t good alternatives, though. Normal SAC305 and other similar alloys do work well enough, and can give results comparable to what you get with leaded, assuming you’ve got a reasonable temperature regulated iron, and some flux for rework.
The main difference in my experience, is that it’s easier to get bad results with lead free if you’re doing things wrong. 63/37 is very forgiving.
Most obviously. If you don’t solder quickly enough and boil off the flux, lead-free solder will take on a kind of gooey consistency. Instead of the surface tension pulling it into a perfect fillet or ball when you remove your iron, it’ll pull away like a gob of soft-serve ice cream.
Leaded will do this too, but it takes an order of magnitude more abuse before the results start getting bad. With lead free, even touching up a joint that you’ve already soldered will often get ugly, unless you add flux.
So, really, there’s not much difference, but lead free makes bad soldering habits more evident.
All of my projects tend to use .5mm pitch ICs, and occasionally even leadless packages with a goofy footprint (to make them hand solderable), and I’ve done them with a soldering iron (no hot air/oven etc) using either leaded or lead free without problem. If you’ve got some reasonable experience soldering, I don’t think you’ll have a problem.
If you’re a beginner, maybe stick with lead for a little while.
Also, very important, is to buy your solder from a trusted source. Lots of the cheap stuff from all the random corners of the internet is incorrect alloys, or with bad flux, etc. It’s not unusual to get something completely unusable (and maybe not even lead free)
It might cost you $70 or more for a pound of good lead free solder, and that sucks, but it’s probably better than buying 4 rolls of random trash for $20 each before you find one that’s usable.
Even with lead based solder, you notice clear differences between cheap and expensive solder.
Oh, i wasn’t ready with typing yet, i hit a hotkey that automatically submitted it :O
Anyway, i have a kilogram roll of cheap chinese lead based solder. Bought it a decade ago for 15 euro, so that’s extremely cheap.
For general use it works fine. But i was doing some more demanding stuff (sealing up a literal can for RF shielding an inverter) and it just wouldn’t stick to the can properly.
I also had an old roll of Multicore solder. With that stuff, i sealed it up with no problems.
I can totally imagine that with lead free solder, those differences are even bigger.
Lead is though a surprisingly useful material in some applications.
Lead bricks for radiation shielding is fairly practical.
As solder, 60/40 does a very good job.
Though, most commercially available lead free solders contain 95+% tin… Tin whiskers practically “require” tin concentrations above 95% to form… So can someone make lead free solder that doesn’t contain 95+% tin?
(Sn90Au10 would be decent, if it didn’t contain 10% gold… But its melting point is 217 C, so that isn’t bad.)
SAC405 is borderline.
KappAloy9 is Sn91Zn9 and it solders to aluminum as well. Melting point 199 C.
Other than that, you have to go to very expensive and exotic alloys.
Thing is, if it’s not bismuth, antimony, or lead, putting in more additives like copper, silver or zinc causes the low temperature behavior of the solder to get worse.
But lead acid batteries are easily recyclable!
That is sure true.
And some of the more sturdy batteries around. (if only they had balancing circuitry for the cells in them too… (always once cell that dies first…))
Though, most recycling facilities likes to be very hands on with the molten lead. (Even if one could just chuck them from a height and then just separate the plastic from the metal before shoving the lead into an oven with a large scraper… Literally, one never needs to even touch the lead with one’s hands… But making simple logical improvements is beyond some developers, especially within commercial environments. “If it works, why fix it?” is a stupid philosophy at times…)
Oddly enough replacing my UPS battery today. It’s most likely an economic decision than a “we’re not smart enough to understand things”.
It is likely partly an economical decision, but likely also held back by the desire to not change what works.
After all, making changes comes with both risks and costs, and honestly, even looking at a potential solution might at times cost too much to be worth while as far as some companies are concerned.
They’d probably last a lot longer if they had balancing as most lead acid batteries I replaced had a dead or shorted cell.
It should be noted the cobalt in most lithium ion batteries is not exactly nice stuff and they’re rarely recycled at a this point.
The other major failure is the sealed especially AGM ones drying out.
“Recyclable” does not mean “recycled”. A lot of batteries are simply thrown away.
The electronics industry has spent tens of billion of dollars trying to replace lead and comply with RoHS, which has amounted to saving less than a couple percents of the amount of lead that is released into the environment from discarded car batteries. Batteries are exempt from RoHS.
Not to mention ammunition.
And all the products not intended to be sold in the EU that use leaded solder regardless since it is cheaper.
RoHS does stuff, but it is remarkably ineffective at reducing e-waste.
In some cases it increases e-waste and pollution BGA failures come to mind.
I don’t think the people who came up with the rules on RoHS solder were engineers or knew anything about mining or the other metals as it just replaced one form of pollution with another.
This may be a case were actions to solve a problem may have done more harm than good.
I melted over 4000 lbs of lead last year. I’ll give you all a health update in 10 years f I’m still around.
I’m adding a google calendar entry to check this page in 10 years.
If there’s no update just assume I’m dead.
I can’t do that Dave..
https://en.wikipedia.org/wiki/Lead%E2%80%93crime_hypothesis
“Alternatives for lead are steel, tungsten-nickel-iron, bismuth-tin, and tungsten-polymer.”
For bullets? In the US most common lead alternative for bullets is copper (nice terminal performance despite the cost) or mild steel (cheap imported ammo), or jacketed hard steel cores (cheap AP) for mil use. I’d expect mild steel to take the crown if lead is banned.
Yeah, when I read ‘tungsten’ I thought “what are they hunting, wild tanks?”
Just wait till Skynet becomes a thing.
Armored delivery drones. ;)
Incidentally, full copper (bronze) ammunition is considered armor-piercing by US law, except for ammunition which the government says is “primarily intended to be used for sporting purposes”.
It’s a fairly complex matter, but it can be simplified down to “If it can be fired out of a pistol, it’s illegal”. That’s why forcing lead-free ammunition is making the sale and purchase of many types of ammunition illegal – except by special government decree. This is basically a form of gun grab by making ammunition instead of the guns illegal to obtain or make.
I hadn’t heard of tungsten-polymer as a lead substitute before but it would probably be a pretty good option, at least as far as density goes. I imagine the wear on the barrel from using an unjacketed bullet would be greater than a more traditional design so that would be an issue though.
There are a few companies already making a copper-polymer non-expanding projectile that uses channels on the bullet to generate a kind of hydraulic displacement rather than the crushing and tearing of a standard expanding bullet design, ARX is one of these. These are usually light weight resulting in higher velocity, which is generally good and results in lower recoil and more energy on impact, but it causes accuracy issues as the point of aim can potentially show a radical shift even over a few yards. A bullet closer to the standard weight and velocity would hit more to the point the weapon’s sights were designed for but then again these new bullet designs would have a more limited effect without that extra velocity to push material away from the channels.
Personally I think lead is going to stick around for some time yet as it is just too good a material to make a bullet with; it is dense so has good weight for size, it is ductile so deforms to bleed energy on impact, and it is cost effective. Sure copper is not bad but is more expensive and some of the new bullet designs and material seem to work well but may not be as reliable or accurate in the hundreds of millions of firearms already out there designed for more traditional ammunition. You would have trouble selling a weapon designed and sighted in for this newer generation of ammo as long as that ammo is still relatively new to the market and not available in the same quantities and price of the old stuff. You also have to deal with the fact many gun owners are fairly conservative what they are willing to spend their money on and have a “if it aint broke don’t fix it” mentality so anything too revolutionary in design or material will not be trusted and so won’t sell well for some time to come anyway.
It’s the age-old energy vs impulse question.
One disadvantage of lighter but faster bullets is that the ballistic coefficient is worse. It loses energy faster, so the stopping power at a distance is less. This is because the bullet loses energy in velocity cubed. The time to target is proportional to v while the energy lost is proportional to v^3 so if you double the speed, you reduce the time to target by half, but increase the energy loss over the distance by a factor of 4.
Another thing is the magnus effect. Since the bullet is spin-stabilized, it also gets a sideways force from air friction. This causes the bullet to travel in a spiral that gets wider the further it goes. The faster and lighter bullet gets a higher rate of spin, and since it has less mass to accelerate, it spirals off the target a lot sooner than the heavier bullet.
There’s all sorts of gotchas.
A scary side note on lead bullets and shot… there used to be a shotgun factory in my home town (Ithaca Gun) and they used to test-fire every gun they built a few times to test it and sight it in using a firing range in the woods behind the factory. in the late 1990s decades after the factoey had closed they did some soil sampling and immediately cordoned off the area to mount a cleanup because some of the soil samples came back as 25% lead by mass (!) and it was right at beside Fall Creek which proceeded directly to the lake from which our drinking water was taken. The fact that they could just pump lead into the ground like that for a century shows how little the US took the dangers of lead seriously before the 1970s.
Maybe not as scary as your location, but a local gun range moved a few years ago. I wonder how much lead could be removed/recovered from the old berm the members had been shooting into for years.
Ah yes.. Over near the Whittier Jr. College, there is a Police Academy building with a “Gun Range”.
If you draw a straight line past the end of the target area, it points to the 60 freeway were it crosses the 605. Many times, workers in that area hear bullets whizzing over head. It seems there are as many holes in the overhead as in the target area. Damn rookies..
I wonder if it would be profitable to recover the lead in the berms of firing ranges.
It is. It’s being done.
Fortunately, the lead is not that soluble once it oxidizes. Unless the soil is very acidic, you probably had nothing to worry about.
They still do it, just up the block in Lansing. Look at the salmon creek rod and gun club, and look at where salmon creek is. BTW, nice to see another Ithican on here.
Still not as scary as teraethyllead as solid metalic and oxide lead tends to stay put .
Metallic lead is harmless enough people have survived for decades with bullets in them without showing signs of poisoning.
But leaded gasoline gets burned inside an engine so you end up breathing it in.
I suspect many of the refineries that made it are still contaminated with dangerous forms of lead to this day.
Now lead shot is more dangerous than bullets as water foul have a tendency of eating it where it sits inside an acidic environment with some rocks and gets ground up.
While we’re on the subject, depleted Uranium makes great projectiles!
(Except with even more potential health problems than lead…)
Locally while as with every where else. the high cost of the “core” deposit while purchasing LA batteries insure recycling of those batteries.However here there there still may be plenty of LA batteries in farm machinery grave yards for, metal salvage operations to seek them out, most likely that existence of LA will be depleted soon. After salvaging what I wanted out of electronic equipment I put the hulls aside until I need to make run to the county landfill. Last time I made such a run I was instrctedto dump the entire load in the pit, because it’s no longer cost effective for the county to process e-waste.Hopefully the pits bentonite clay seals are in good shape. Where leads pipe may in service for potable water supplies, I hope the lessons learned at Flint Michigan aren’t lost, on other municipalities.
“insure recycling of those batteries”
Not really. It gets baked in to the much greater price of products that contain lead batteries, such as cars and motorcycles, and when it comes time to replace them, many people just toss them.
It’s especially true for used cars. The first owner paid the recycling fee, the last owner didn’t. They just see an old dead battery and think “Hmmm… do I spend money on gas to take this to a recycling center, or do I just chuck it behind a shed?”
Thank you for fanning the flames of fear in dumb people. First it was mercury, now it is lead.
I had a girlfriend who worked at a manufacturing plant that did a lot of small run and very customized devices. She stenciled the solder paste onto boards, she did hand pick and place, she did touch up, among other things. The company had mandatory lead screenings for everybody. She was one of the people with the most workplace contact. The results of the lead testing were interesting. She had undetectable amounts. Apparently wearing clothing, latex gloves, and not dipping your potato chips in the lead paste is enough to keep you clean. What was interesting is that a bunch of the men in the engineering group all had high levels of lead. They have far less workplace contact, but they all shared a hobby: They all shot together at an indoor shooting range. Now they could have been dipping their potato chips in the lead paste at work but the more popular theory was that in the shooting range the explosion driving the lead out of the barrel vaporized enough lead that it contaminated the air. They may also have got more lead on them cleaning the guns after using them. In any event, from personal experience, I don’t worry too much about my electronics use of lead. I do wear gloves when I clean my guns though, and I have never shot in an indoor range.
The problem with lead in electronics isn’t at the beginning of the life-cycle, as you say it’s very easy to take adequate precautions. The problem is at the end of the product life-cycle, when devices are thrown away, they’d leech lead into the soil and into the plant and animal life. It’s perfectly sensible to prevent that from happening by preventing the use of lead where it’s not needed.
The problem isn’t that there is lead in the products.
But rather that very few countries in the world even have something resembling proper E-waste management.
And that few companies build/design their products with easy recycling in mind.
The argument that “lead leeches into the soil over time” is mostly showing how primitive most modern societies are.
Especially considering how proper recycling isn’t actually that hard to implement in practice.
Fixing the problem of poorly designed products is also simple, the government could just fine the company for it. (and use the money from that fine to pay for the recycling effort needed for said product.)
Though, then there would need to be a fairly large international organization making such legislation, the EU were “close” with RoHS, but they too looked at the wrong side of the problem…. (RoHS fixes the E-waste problem by making the waste a bit less toxic, but states nothing about recycling.)
I disagree with your first sentence, but not your post overall. Lead leeching into the environment is an immediate problem with an immediate win by restricting its use. Of course we should also recover as much hazardous waste from our e-waste as possible. I expect there will effectively be mining in landfill areas in the coming decades.
I would be partly surprised if land fill mining doesn’t become an industry at some point or another.
After all, the concentration of things like copper and gold would likely be fairly high compared to other sources.
But it is rather silly that the solution to e-waste at the moment is to ask the question if it contains x materials or not. Instead of doing something about the fact that millions of tons of e-waste is simply dumped.
The US at least ensures that their land fills have clay around them to stop the leeching of substances into the local ground water. But a lot of other countries in the world simply dumps it in Africa and other less economically well of places. (These places though do get “payed” for it. But this is more figurative then anything meaningful.)
It wouldn’t be all too unreasonable to spend effort into setting up recycling initiatives, and subsidize local recycling efforts, and also fine corporations that actively works against the ease of recycling of their products.
Not to mention how that can help countries have more self sufficiency in their economy.
Even if you design the products to be properly recycled, that does not ensure they actually do end up in recycling, or that someone doesn’t ship them to Africa because it’s cheaper.
Yes, a more easily recyclable product doesn’t stop it from getting shipped of and dumped elsewhere.
Though, at least it is a step in the right direction and forbidding companies from intentionally making their products harder to recycle would keep the recycling initiative in mind to a degree.
And the countries that imports all this e-waste do get a little bit of an easier time to handle it. Since it is resources that they could use to boost their economy with.
But yes, ease of recycling isn’t a solution in and off itself. Its just one piece of a larger puzzle.
This makes Apple one of the most hypocritical companies when it comes to protecting the environment. They tout how “green” they are, while most of their current products are glued shut, with the batteries glued in place inside to make it extra difficult to take apart.
The cost to destructively disassemble these products to remove the batteries so the rest can be safely shredded exceeds the value of recoverable metals. It’s even worse for carefully taking them apart to recover some parts intact for repairing other devices.
So a guy like Louis Rossman can repair your Apple device that has a design flaw which leads to a high failure rate, but he’ll have to very carefully pry the thing apart, and the replacement component will have to be expensively recovered from another device that’s failed in a different way.
If your difficult to repair Apple device is more than a couple of years old, it’s not really worth the cost to repair, unless there are files on it you have to recover. Seems to be why Apple pushes their cloud storage and other online services so hard – they know almost everything they’ve sold recently is a brick waiting to happen, and when they die they want you to just toss it, buy their latest gizmo, then login to your iCloud with your AppleID and there’s all your stuff.
That’s the server-client computing model that held sway for the first 30-ish years of computer technology until the rise of the personal computer in the late 70’s.
>”the countries that imports all this e-waste do get a little bit of an easier time to handle it.”
The reality of the matter is that the easiest way to “deal” with the waste still is just chucking it in a pile and burning it, then sifting through the ashes for the metals. Even if you make the products easier to pull apart and separate the different wastes, the poor countries that accept the waste are doing so because they get paid to accept it – they do not get paid to actually recycle it. They’re merely the lowest bidders – who demands the least money to take the waste.
What happens next is, they don’t even attempt to recycle the waste. They simply take it from the port to an open dump and leave it there. This is cheaper than trying to recover any value from the waste by official recycling practices.
What happens next is, private individuals sift through the waste and basically just burn it to get rid of the bulk of plastics. Even if you make the plastics easy to separate, that’s still extra work compared to just throwing the thing on the pyre. It makes absolutely no difference.
A local indoor gun range was recently built with a LOT of precautions. Double sealed entry/exit doors, HEPA ventilation, peal-able sticky floor tape to catch anything being tracked out, sinks for washing hands/face upon exit; I’m not sure what other precautions are used, but I was unaware of the hazards until they were pointed out to me!
You can also find lead used to make cables more flexible today.
Just to put the lead-based solder into perspective: in 2008 we dropped 12,000,000 kg of lead into the ocean, as shielding for a 580 km cable to Norway. And that’s just one of many cables.
Forget solder.
Look up the history, political entrenchment and profits of tetraethyl lead additives used (and emitted) in gasoline through most of the 20th century, and responsible for the majority of the lead we all carry in our bodies.
https://www.thenation.com/article/secret-history-lead/
Oh man. That definitely gets me riled up.
I have a love/hate relationship with GM. They seem to come out with some amazing products (small block Chevy, the Volt and Bolt), but it’s crap like that that makes me hate them.
Don’t hate the game, hate the player.
Engineering and purchasing demand certain cost savings not always
related to the “Best Interest Of The Consumer”.
A few years ago, a Ford engineer I met relayed his take.
Corporate decisions are “required” and made on anything raising the build price more than $0.05 per unit.
Witness the Chevy Volt, built with cheap steel in the lower kick plate under the doors.
When that Volt was tested by NHTSB, it failed, and crushed the battery.
Battery leaked coolant, created a short, burned a row of crashed car in the junk yard.
The bad press almost killed the Volt then, and today, it’s gone.
Chevy still has an electric car, I think it’s called T ripple A (AAA)
Nearly all the drop in crime in the 1990s was due to the phasing out of leaded gasoline and the legalization of abortion.
Less lead paint.
Abortion rights, so less people were being born to poor conditions that create crime.
The increase in gun ownership and laws requiring granting of concealed carry permits.
My daigjter was servely poison by lead at 70 mg do she is 24.now
Excellent read.
As with most substances (chemicals, elements, compounds, solutions) if you practice proper handling techniques and dispose of with the correct protocols, you’ll do just fine. Unfortunately, at least here in the U.S. we have an emerging trend of “chemophobia” taking place. Mostly, due to our government agencies efforts to restrict access to any and all chemicals for private use. And this has created a general consensus by the lesser informed citizens (chemistry ignorant), that if you enjoy and practice amateur chemistry at home as a hobby, then you must be a criminal. Because, what on earth could you possibly be making other than illicit drugs, right? Ah, then we have the activists (also chemistry ignorant) who feel the need to blow various concerns out of proportion, and this in turn fuels the governments agenda with limiting even more chemical access.
The trickle-down effect has led to various states and jurisdictions passing laws prohibiting the possession of lab equipment, glassware, and chemicals by an individual without the necessary licenses. More scrutiny placed on people who wish to purchase various chemicals for personal use, with an induced feeling that we must be doing something illegal. The lack of decent chemistry sets being sold in stores these days for kids to learn basic chemistry principles at a young age. As well as many schools, mainly middle-school and high-schools changing their curriculum’s in regards to the teaching of chemistry by excluding the hands-on practical labs that parallel the concept being taught in class. Mostly, relegating any chemical reaction demonstrations being performed by the teacher only. This in turn has led to students who decide to study chemistry in college, where most have had very little interaction with chemicals up to this point. So they have more apprehension with handling chemicals while performing practical labs with their classes, which can discourage and cause some to re-think their chosen field of study. This disturbing and wide-spread dysfunction of students awkwardness/fear of the handling of chemicals has been observed by many professors at universities and independent labs who employ college interns. Where does the U.S. rank with the rest of the world concerning chemistry performance? I don’t know exactly, but I do know we are way, way down there!
So, with my diatribe and tangential path I took outside the scope of this article, let me sum it up like this…
Lead-free solder SUCKS! It does not wet as easily as 60/40, and creates more crystalline and brittle joints. I’ve personally seen an increase in consumer electronic devices failing from a bad (Pb-free) solder joint, especially with suface-mount components detaching from the solder pads on the board. Lets not even mention the crappy defect-prone BGA style footprint chips. Also lead bullets are superior to any of the Pb-free alternatives being offered today. Bismuth made ammo are more expensive, steel and tungsten-alloy ammo causes much more mechanical wear on the internal surfaces of the guns barrel. Lead exposure from game-meat? Yeah right! I don’t know about anyone else, but I harvest the meat from my hunted animals very shortly after they’re killed. Any fragments of lead from the kill-shot have not even begun dissolving or diffusing into the harvested meat. Maybe I can see possible lead exposure from buckshot in hunted water foul, but not likely.
As far as our agricultural practices in this country are concerned, you should be more worried about the massive wide-spread use of glyphosate being applied to our crops, namely corn. Organophosphates are uber bad! They make kids stupid, cause all sorts of cancers and contaminate everything in our environment. Not to mention that nearly everything that Americans eat is corn-based or has been processed with a mutlitude of corn-derived additives. Yep, even the meat we eat is essentially derived from corn! Cows are not able to sustain themselves on a corn-based diet naturally. They are grass eaters and have evolved to this day as such. But at the massive CAFO’s, the cows are fed corn-rich diets, which actually makes them sick. So they are given special medications to help counteract some of the symptoms brought about from their corn diet. This only sustains them for a specified time period up until they’re slaughtered. If they were allowed to live any longer, they would become too sick and die anyways from systemic issues caused by the corn in their diet.
Yeah, worry about that! Oh and try not to breath in deeply around Pb dust…
Finally, some one with their head on straight! Thank you.
+1 this post the best way to get kids to want to learn about science is hands on lab experiments and if they can’t do that you have less people becoming interested in it.
Then again, you don’t have to do it like the chemistry sets of the early 20th century, which were literally explosions waiting to happen – or things like “how to make cyanide gas in three easy steps”.
There’s lots more to chemistry than making things go bang and fizz.
Pretty good up till the glyphosphate rant. I knew chemists in school in the 1970’s who were absolutely positive we were all going to die from the dioxin byproducts in commonly used pentachlorophenol. The slip-n-fall lawyers lusting after Roundup money from moronic juries are pushing the glyphosphate narrative hard. There is such a thing as recognizing an impossibility.
60/40 eutectic solder is quite resistant to tin pest formation, unlike many of the lead free solders. The amount of lead used globally every year in solders is exceeded by that used in ammunition ( ~4% of total production, iirc), to put things into perspective.
I have certainly noticed premature failure in consumer devices using lead free solders, which means the entire device will likely end up in land fill prematurely, leaching whatever exotic trace metals and transition elements the lead free solder contains.
Studies have shown that lead levels in hobbyists that solder or in workers doing electronic assembly work are not particularly elevated compared to the general population, and solder quantities used on boards have dropped dramatically since the valve based point to point wiring of the pre transistor/IC age. As discussed, simple hand hygiene is adequate when soldering to minimise ingestion.
Arguably, a more sensible solution than eliminating lead from solder is to have effective e-waste recycing.
Yes, it is fun how the solution to e-waste currently is to say, “well, it isn’t a problem if it doesn’t contain lead, cadmium, etc.”
It is still many millions of tons of e-waste each year literally getting dumped.
Setting up recycling initiatives and subsidizing local recycling efforts isn’t hard as well.
Banning the use of lead in solder because it can leech into the environment is fairly silly, one should instead work on not dumping millions of tons of e-waste into the environment.
The environmental impact of dumping millions of tons of electronics is likely not greatly impacted by the electronics containing lead or not. And a lot of third world countries where a lot of e-waste gets dumped, generally are more concerned with the other downsides of e-waste. People getting cut on sharp metal cases, PCBs, and other crap and then getting serious infections is likely a bigger and more direct issue than the lead content.
I deal with the recycle community all the time. (Here in California) You may be amazed to discover most components are recycled. Usable hardware, recycled and resold. Witness the massive Ebay stores as well as retail/wholesale distribution. By buddy Chuck has a cute little Mercedes with the plate number “GO 4 AU” in reference to all the gold he extracts from the scrap. The real bundoggle is the can and bottle recycle program that is under water and destined to fail.
Yes, but when he extracts the gold by separating it with cyanide, what does he do with the remainders?
Picking a nit: Tin Lead eutectic is Sn63/Pb37. Sn60/Pb40 is close, but not quite.
Yes, and you won’t get cold joints with the eutectic alloy.
Thank you Maya, this was a very interesting and informative article.
“Back in 1983, the US Navy commissioned a study (PDF) to examine just how pervasive lead contamination was in an environment where lead-based solder was being used for soldering work.”
Yes, and conclusion #1 on page 12 states:
“No significant inhalation hazard from lead fume exists in soldering and pot tinning environments. In addition, lead fume is not a significant source of surface contamination. The practical implications are that mechanical exhaust ventilation and physical isolation of soldering areas are not essential to prevent a lead hazard. (Irritating and/or toxic decomposition products of flux may require ventilation, however.) Lead contamination may be spread to adjacent areas by accumalation of dross dust and/or solderers’ contaminated hands”
The WHO limits back then were 3000µg/week. Up until 2010, WHO’s guidelines for lead were 25µg/kg of body weight per week, so for a 180 pound person, that equates to roughly 2000µg/week, only a 33% reduction from the value in the Navy’s study. The current WHO guidelines removed that standard in 2010,based upon the conclusions of the JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES, Seventy-third meeting, Geneva, 8–17 June 2010. Specifically, stating:
“For adults, the mean dietary lead exposure estimates range from 0.02 to 3.0 μg/kg body weight per day. The lower end of this range (0.02 μg/kg body weight per day) was considerably below the exposure level of 1.2 μg/kg body weight per day, calculated by the Committee to be associated with a population increase in systolic blood pressure of 1 mmHg (0.1 kPa). The Committee considered that any health risk that would be expected to occur at this exposure level is negligible.”
In regards to dietary lead exposure, lead contamination from soldering would be including in these calculation because the main concern would come from ingestion.
Also, in my opinion, putting the Navy study together with the 2010 JECFA meeting will tell you that you have bigger concerns coming from food you intentionally ingest as opposed to soldering.
“In civilized nations …”
This should have been said more politely (i.e., in a more civilized fashion).
Hard to see how you could have a nation (is a stable community of people with common culture) which was not civilized (at an advanced stage of social and cultural development; Which isn’t the same thing as technologically advanced).
If anything, it seems like the more technologically advanced (cities, factories, etc.) societies may be more prone to lead exposure in the workplace (requiring regulations, etc. to reduce such exposure).
What did you have in mind for uncivilized nations?
There are places where lead and/or mercury are naturally present in pure metal form. Are we supposed to “clean up” such sites? Near where I live is a mercury mine, which has been shut down for several decades. At its peak it was the largest producer of mercury in the USA, perhaps 2nd in the world.
Go gold panning in the mountain streams of this part of Idaho and chances are you’ll end up with a little bit of pure mercury in your pan, naturally sourced from deposits of cinnabar. If you do find mercury while panning for gold, you want to save it because it may be amalgamated with gold.
The old school way of getting the gold out of the mercury was to put the amalgam into a small iron crucible placed over a fire hot enough to vaporize the mercury. Of course you’d only do this on a day with a pretty good breeze and stay plenty far away, upwind. After the mercury is gone you have a lump of very pure gold left behind.
Mercury amalgamation was also a way to pick up the finest gold dust, and to extract gold from “black sand”. Put all the fines into an iron skillet (never to be used for cooking!) then pour in some mercury. Use something made of glass, like a mason jar, to grind around and around until the mercury soaks up all the gold.
There are other ways to separate out and consolidate fine gold, or to separate it from mercury without vaporizing the mercury into the air.
Is the straw man an artifact of mercury poisoning?
“70 years after France”
well, not really. It was forbidden to use white base lead paint since 1915 for professional painters, 1948 for all professional use.
Anti corrosion lead paints where not banned until 1993, so pretty much all painted ironworks made before this date are suspicious, yet nobody take care of correct handling.
With the vent fans, nitrile gloves, breathing filters, awareness of the slightest potential problem exposures, increases in air and water quality, etc. I expect this generation to have a noticeable increase in lifetimes and IQ and decreases on geriatric diseases. Anything showing up yet?
Would be nice if there were more cost effective and required impurities testing on people as standard diagnostics prior to prescribing therapeutics just to be safe. I’m amazed at how even when requested in my experience, the testing isn’t performed like something is being compounded and concealed up through the professional degree’d and licensed rackets networks.
Then again, I’m in Michigan and from what I’ve observed forensics is only performed if the State is masked (seems like meets definition of spirit and intent of the code even if gross frauds and cheats) armed robbing their victims. With all the drug testing and other labs capabilities to perform; seems hair, urine and blood testing can be performed more cost effectively… especially since being subsidized at many of the locations.
Another disturbing observation is the agriculture laboratories not as a public service performing thorough testing of agriculture, food and drug products. Appears there are areas of opportunities for improvement in not only the cost effective basis ways.
Strange how poisonous the culture is more than I realized growing up and seems historically is in a worsening cycle too.
Some thoughts on this. First of all after 35 years of daily exposure to lead solder in manufacturing and 20 years of being next to a target range I decided to have a heavy metals test during my physical last year. Also was concerned about other metals I come in contact with regularly. All came back normal. Even my hydrocarbons which I thought were going to be sky high from defluxing PCB’s daily was nothing more than a normal person. Turns out you really have to be ingesting things to have them build up and simple measures to protect yourself do work.
As for who still uses lead in solder, essentially it’s only real use apart from making SMT easier to work with is for hi reliability vibrational assembly which we do, hence why we still use leaded solder. Other than that lead well slowly go away. As for environmental exposure battery recycling places are about the worsts. Remember all granetic soils contain lead, so it’s already everywhere. People make way too big of a deal of it.
One thing that has not been covered here is the reliability factor of RoHS compliant solders, such as SAC-305. This common alloy in consumer electronics manufacture is 96.5% tin, 3.0% silver and 0.5% copper. It melts around 223C. Typical SN63/PB37 melts around 183C, and has a pretty clean and sharp melting point. SN63 or SN60 tends to be very forgiving in terms of its soldering characteristics.
The SAC-305 and other high-tin RoHS compliant solders tend to be brittle. This contributes to the shortened lifetime of these devices. Case and point – the Microsoft X-Box “Red Rings of Death” failures. What was happening here was that the solder balls on the BGA microprocessor were cracking. Many of these were due to people keeping X-Boxes in consoles, where they’d heat up. When they were done playing, they’d shut it off and it would cool down. If these BGAs were lead solder, they probably wouldn’t have died. There was one company that was offering a service, where they’d remove the circuit card and reflow the BGA in place.
Again, the fact that SAC-305 and high-tin alloys are brittle will contribute to the shortened life of consumer and other devices. The lead solder is softer, a bit more flexible, and therefore will give a little bit when subjected to mechanical or thermal stress. There is a very good reason why military, space and medical electronics are still required to use leaded solders. That is to improve their reliability versus if they were made with high-tin. While it needs to be responsibly handled, there isn’t a good substitute for lead yet. And there are a number of other metals out there that are being touted as substitutes, such as indium. However, many of these wind up being just as toxic as lead.
There are no easy answers to the problem.
My father was a “Hot Type Printer” after WWII. He operated a Linotype. The pot of molten lead was literally in front of the operator. He fed the machine ingots of lead and it in turn produced lead “slugs”. He handled all of those lead “slugs” that were produced by those machines. He cut and trimmed the inch-thick stacks of these slugs on a table saw, wiped them clean with rags soaked in carbon tetrachloride while he smoked Lucky Strike or Pal Mall cigarettes with no filters, of course. I distinctly remember seeing his blackened fingerprints on the cigarette paper. Carbon tetrachloride was the solvent of choice because it was non-flammable. After the print job was done, all of those slugs were dumped into a big pot or cauldron and melted down to produce more ingots to feed the linotype machines.
He complained a number of times of seeing smoke in his peripheral vision there were many times he had blood in his urine.
All those years of handling the lead, breathing and ingesting the dust and vapors took its toll. He developed diabetes at 35, started having chest pains at 45 and started taking nitroglycerine at 52. Was it the lead, the smoking or the carbon tetrachloride solvent?
There is more. At the age of 59 he had to quit his job at the print shop. He also quit smoking. Shortly after that he underwent quadruple bypass surgery. At 62 I caught him taking nitroglycerine tablets for chest pain. After his 64th birthday he was declared to have kidney failure and was scheduled to get surgery for a dialysis fistula. The surgery did not happen as scheduled. His blood pressure and vital signs were off the charts. He was transported to a hospital that placed a “tap” through his chest wall and into his heart. Hemodialysis was started as soon as the “tap” was installed. He did not live to see his 65th birthday, his heart stopped during the last 5 minutes of his last 4-hour dialysis treatment.
After he was cremated, I removed a few small bone chips from his urn. The bone chips were dissolved and analyzed by ICPMS for lead. His bones were loaded with high amounts of lead. Was it the lead, the smoking, the carbon tetrachloride solvent or all of them?
Seems everyone forgets that AV Gas still has lead in it. Along with most planes having no emissions controls at all!
As I recall, my airplane used unleaded Av gas
Yore argument is invalid.
Does anyone know what level lead melting pots risk is? I have heard that fumes over 650 degrees C give off partials however most melting pots work at much lower temperatures. We have lead melting pots at 450 degrees C is there really a risk of lead fumes and what is in the lead fumes ? Lead is heavy metal so I cant see fumes having partials at this lower temperature and I am wondering what is actually thought to be in lead fumes from a melting pot. We wet scub our fumes and I am wondering about the effectiveness of what we do, the first step is to understand what is in lead fumes. There seems to be little or nothing on lead fumes from melting lead. In this article they say that lead partials are so fine you cant detect them. Any thoughts or information is welcome, my thoughts are it could be from nothing to high risk if not removed but is seems to be that if there is little to nothing then efforts around this should be normal but if at lower temperatures more system checks should take place.
In some cases you can melt lead at lower temperatures but sometime people like to make fire in lead to burn off dross , lead oxides, which I think is risky.
Please commment
Lance Castignani