They always told you not to mix water and electricity. And while yes, that is good general advice regarding the two, you won’t rip a hole in the fabric of space-time should you go about it responsibly. Water will conduct electricity, so why not use it to switch on a lamp?
[Manvith Subraya]’s Hydro Lamp is, among other things, a reminder not to let Big Switch dim your idea of what’s possible with simple components. Switches don’t have to be complex, and some of the most reliable switches are pretty simple — the reed switch and the mercury tilt switch are good examples. By salinating the water at a ratio of 1:1, [Manvith] ensures power will flow through the acrylic tank, completing the circuit and lighting the 20W LEDs in both ends.
The brief demo video after the break sheds light on an interesting aspect of using water as a tilt switch — it’s not instantaneous. As he slowly moves the lamp from vertical to horizontal and back again, the light brightens and dims with the tide of electrons. We think it would be interesting to build a motorized frame that takes advantage of this for mood lighting purposes, especially if there were a few LEDs positioned behind the water.
Water is often used to explain the basic principle of current flow and the relationship dynamics of voltage, current, and resistance. As we saw in this water computer, the concept flows all the way into logic gates.
http://www.instructables.com/id/Hydro-Lamp/https://www.youtube.com/watch?v=2abCLPwsYJc

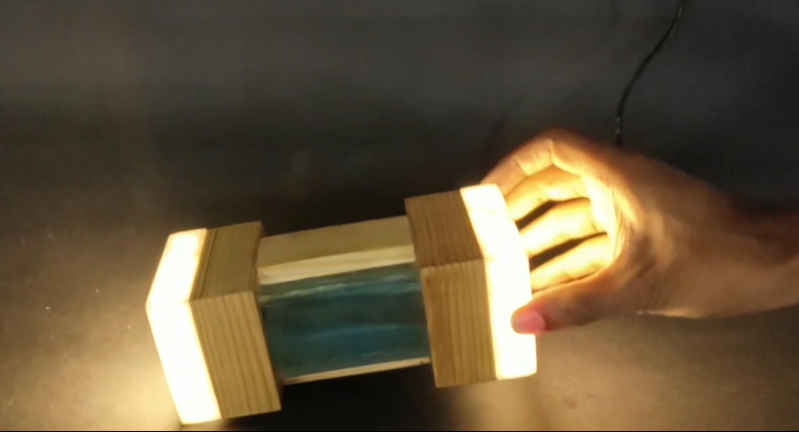













The current flow is instantaneous in the circuit. It’s just that the water is acting as a variable resistor in the circuit -the thinner the cross-section at one side, the higher the resistance.
Nice
My one question is safety: As the current runs through the saltwater, you’re effectively electrolysing it. As it runs, is there anything preventing a buildup of hydrogen and chlorine gas inside the sealed container?
A very sensible thought but it should be fine as is – being sealed and relatively low power any gases generated should diffuse and recombine in the water. If it was higher power, vented to prevent risk of over pressure (probably implying high power) or producing corrosive to the container gasses then you could have trouble with harmful emissions. But I don’t see any problems here.
Being a closed loop (as long as the electrodes are not being decomposed – so perhaps graphite?) it should be perfectly safe for extended use. And even if the electrodes are be being consumed I would say its no safety risk just won’t work forever as eventually there won’t be a complete circuit.
Yeah it’s surprising how well water dissolves a gas into itself, particularly when there’s something reactive with that gas sitting in the water… For example, put some iron or steel hardware in the bottom of a plastic bottle with an inch or two of water over it, and seal the lid, watch over a few days as the bottle collapses from the oxygen in the air dissolving into the water and making iron oxide on the iron/steel. It’ll keep going until either all the free O2 is gone or all the iron is gone.
Gases may redissolve into water, but that is not the same as oxidising H2 or whatever and getting the water back.
More importantly: if the current is low enough, there should be no gas generate at all.
As its a closed system the gasses will diffuse and restore to the most stable configuration for those chemical components under those conditions. So any sodium deposited will eventually reclaim its chlorine, hydrogen its oxygen, so even if there is enough to generate gases it should form an equilibrium of higher gasses while on and just return to salty water when off. (assuming it doesn’t interact with the electrodes and form something more stable which it should not…)
And before some smart guy points out salts dissolved turn into free ions in water so what I said was not technically 100% correct it is close enough.
You won’t get sodium unless you do electrolysis on molten salt.
Electrolysis of salt solution would get you hydrogen, chlorine and sodium hydroxide.
http://www.docbrown.info/page01/ExIndChem/electrochemistry03.htm
@tekkieneet You are correct – though can get sodium deposits as the water is consumed and it becomes too concentrated to remain in solution. But I was trying to keep it simple while making the point the whole system is going to try and maintain an equilibrium rather that being 100% precise (as that would be really really tricky)
To be super precise with how stable elemental hydrogen and oxygen are technically the air in that container would after being run be richer in them for example, and as Hydrogen pretty much can’t be perfectly contained over a prolong period that tank would become very rich in O2 and start lacking in water…
This isn’t a closed system because energy is being brought in by the current. This drives the system out of equilibrium.
What can also happen is the hydrogen combines with the oxygen present in the volume of air inside the box, forming water. Then the oxygen in the container will be slowly replaced with chlorine gas, and the water turns into a solution of salt and sodium hydroxide.
@Luke yes, and no. The wonders of language is that it is closed – the cell does not change in elemental ratio’s inside – its not taking on new ions from the surroundings etc, but as you say the input of energy pushes it towards a new equilibrium in how those elements are arranged. So this cell is closed in the same way a ‘sealed’ lead acid battery is chemically the brew is all still there nothing added or taken by its use.
But I think we can all agree no matter how we phrase it that at these low powers this is perfectly safe, and baring a poor choice of electrode should work for quite some time – Which is the point, the point most certainly is not exactly how to describe in concise discussion friendly format the chemistry inside – its not like we want the PHD thesis studies that probably exist reprinted here.
Basic hygiene recommended
The last time I had to make water conductive I ended up using copper sulfate instead of table salt. It just doesn’t work as well as you’d like.
Killer idea for the Darwin Award competition
:o)
A motorized frame something like this wave machine?
https://www.youtube.com/watch?v=H2dzy2m0JGo
Also how long before the salt water corrodes the electrodes?
Platinum is expensive even plated copper. Carbon is a good second choice. Old carbon zinc D cell batteries, a pair of pliers and a careful but firm extraction means they are pretty much free.
If the voltage and current is nice and low and you sense with short pulses, you’ll be able to measure the resistance without any issues with hydrogen and oxygen forming. Hydrogen is entertaining. Even in small quantities, if it’s enclosed it can produce quite a bit of force. As a child I used to make four litre bottles into hydrogen rockets, coiled sandwich bag ties make great igniters. Definitely woke you up. A small sprung pressure valve probably wouldn’t go a miss on this thing if it’s going to be in long term continuous use.
Hydrogen, mixed with oxygen, sure can make quite a bang.
https://www.youtube.com/watch?v=l9CI6KSV560
Without dissimilar metals that favor corroding the copper it will take quite some time till copper will be bothered – the problem boats have is forming crappy batteries with the sea water and different metals. There are a great many very old copper/copper alloy finds from the sea that are relatively untouched by the years. Same is true for old ship hulls and we all know iron/steel rust rather readily – not a huge amount of available oxygen dissolved in the water, so without the extra help of dis-similar metals it lasts rather well.
(In this case assuming 100% airtight the copper should never just corrode – there isn’t enough oxygen in there to corrode much of anything – even an iron nail would probably only get somewhat pitted if you stuck it in there)
“there isn’t enough oxygen in there to corrode much of anything”
I think you missed the 1/3 of the atoms in the big transparent mass inside that. Water is pretty stable, but that doesn’t count when you are actively electrolysing it.
Aye the current will do the work with water, but the question was saltwater corrosion which I took to mean how long will it last in ‘off’ positions. Not if it will last in use. Certainly not the right choice of electrodes for longevity of use. Though with the stated Wattage based on my electric etching plays I’d say it will last rather well, certainly not weeks of current flow but still a pretty long time.
“Also how long before the salt water corrodes the electrodes?”
Assuming it’s around 12V going through there, and seeing he just stuck the stripped cable in there, I’d say it’ll last a few dozen hours, maybe less.
Salt water alone would corrode it pretty fast, but running current through it will be running reactions all the time on those tiny copper strands… Maybe I’m being too optimistic and it won’t even last that dozen hours XD
One nice suggestion I’d make would be to add some highlighter ink and a UV led at each end. That could make for a pretty interesting effect.
I like the idea of the switch better than the idea of also using it as a power conductor. I would definitely want to add a transistor.
But the “knock it over to turn it on” part of the design is great! Huge improvement for when you’re searching for the lamp in a dark garage.
I just want to be pedantic about the wording of “Water will conduct electricity…” While we all already know that pure water won’t without caveat, I can’t help but think this wasn’t the best choice of wording.