Ventilators are key in the treating the most dire cases of coronavirus. The exponential growth of infections, and the number of patients in respiratory distress, has outpaced the number of available ventilators. In times of crisis, everyone looks for ways they can help, and one of the ways the hardware community has responded is in work toward a ventilator design that can be rapidly manufactured to meet the need.
The difficult truth is that the complexity of ventilator features needed to treat the sickest patients makes a bootstrapped design incredibly difficult, and I believe impossible to achieve in quantity on this timeline. Still, a well-engineered and clinically approved open source ventilator might deliver many benefits beyond the current crisis. Let’s take a look at some of the efforts we’ve been seeing recently and what it would take to pull together a complete design.
Bag-Based Ventilator Designs
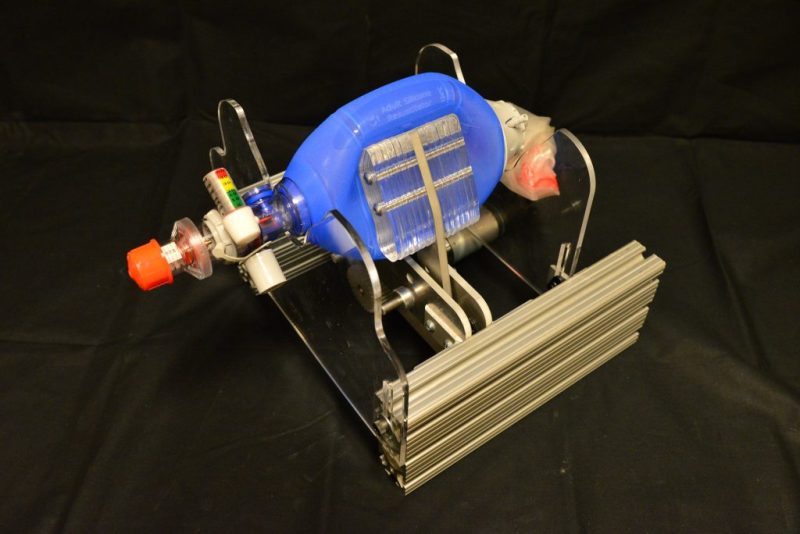
We’ve seen a number of designs based on a bag valve mask (BVM), also known by the brand name Ambu bag. You’ve likely seen these in medical scenes on television where a large flexible bladder is squeezed by a medical worker to push air into the lungs of an unconscious patient. Many recent DIY designs work by automating the squeezing of this bag. This does the work of a BVM, but I hesitate to call them a ventilator because they lack many critical features. I’ll address those below, but it’s also worth your time to watch this fifteen-minute video detailing the topic:
The use of a BVM is most often found in short-term situations where a patient with otherwise healthy lungs needs to be kept alive until they can be transported to a proper ventilator: think of a 20-minute ambulance ride. These are not designed to be used for long periods of time and we’ve seen anecdotal reports that COVID-19 patients are needing invasive ventilation much longer than expected, at more than a week and in some cases multiple weeks. And then they need to be weaned back off them.
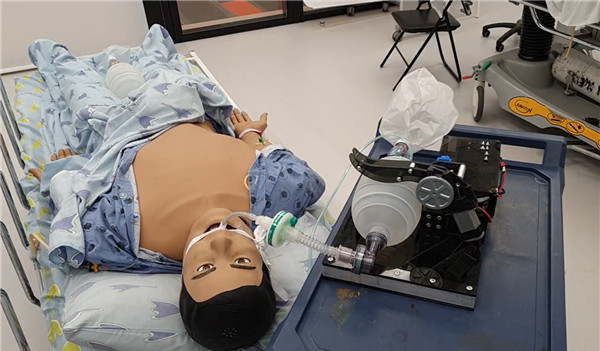
Seen here is the AmboVent design developed in Israel by a volunteer group that included both physicians and engineers. It’s one of the most advanced bag-based designs we’ve seen, yet it raises a couple of concerns. When providing invasive breathing support as shown by this intubated test patient, the air needs to be both heated and humidified — normally a function of the sinuses, which have been bypassed to insert a tube into the trachea. It’s unclear if designs like these can be used with an external humidifying device.
The design also lacks the granularity necessary for the sick lungs of COVID-19 patients. Both the inhale and exhale cycles need to to be carefully regulated and monitored to ensure that as much of the lungs are being used as possible and no damage is being done to the patient’s lungs. Although there is a pressure sensor and “breath profiles” in the software of this particular design, the only control is the rate at which the motor-controlled arm squeezes the bag. There is also no in-built mechanism for regulating the oxygen concentration being provided.
Other similar bag-based designs include the E-Vent from MIT which uses a paddle to squeeze the bag, and the OxVent coming out of the UK which places the bag in a chamber and uses compressed air to squeeze it. All of these designs operate on similar principles and have the same limitations. But by far the biggest limitations are the lack of sensors and the complexity of software. Patients using these machines are sedated and often partially paralyzed. Intensive care ventilators are able to sense air pressure, oxygen concentration, and breath rate and adapt quickly and accurately. Developing these features is a software nightmare for a rushed product. And the lack of sensors to alarm under many possible failure states make these designs something that would require uninterrupted human oversight.
Tesla’s Ventilator Prototype is Closer But Still Far Away
The ventilator prototype demonstrated by Tesla engineers last week is certainly a step up from the state of the bag-based designs. That said, the clever bit of using an Ambu bag is that they’re already at every hospital in the world en masse.
The major advances found in Tesla’s design are the sensing mechanisms for both oxygen concentration and inhale/exhale pressures. As demonstrated in the video, there is a mixing chamber where oxygen is added to ambient air. The system can adjust based on oxygen concentration in the exhale tubing. There is also separate pressure sensing and actuation for both inhale and exhale cycles. Acute respiratory distress syndrome (ARDS) is one of the major challenges in treating COVID-19 patients and granular control of these separate pressures is a key component to treatment.
The sinuses are once again bypassed by this invasive ventilation, but since this design depends on a pump and not a compressed bladder with limited volume, it can likely be used with an external humidifying device. If this system were to be deemed usable it would still need to be manufactured, raising questions of supply chain availability, and the same “software nightmare” mentioned in the previous section will be present here.
Right Now We Have a Supply Chain Problem, Not a Design Problem
If you’re serious about ventilators, you simply must go and read Ventilators 101 by Bob Baddeley. This masterpiece of an article lays out the challenge of designing and manufacturing these advanced machines. These machines solve really hard problems of interfacing with the human body and I think it’s unlikely there are suitable shortcuts around their complexity. The good news is that we’ve already designed, tested, and extensively used them. But we’re having trouble making a lot of them right now.
So our problem right now isn’t ventilator design, it’s manufacturing supply chain. This is being worked on feverishly and by a huge number of people. This episode of the Planet Money podcast follows the sourcing of ventilator pistons. The ventilator manufacturer Ventec is partnering with General Motors who are spinning up their supply chain up to make tens of thousands of ventilators, with the first units planned to be in the field some time this month.
We have a desperate shortage right now and it is really awful. The hope is that the windfall of this supply chain effort will mean an abundance of this equipment. It’s worth mentioning that our most precious resource is not the equipment but the health care workers to operate it. Doing everything we can to support them and reduce the number of people who need care right now is, in my mind, an equally important problem to address. Thank you to these heroes who put their health at risk to heal others.
Long-Term, Does an Open Source Ventilator Design Make Sense?
What if there had been an Open Source ventilator design available in when this all began? Would it have made the difference when China was first seeing severe cases? Would it have made the difference in Italy, Spain, Europe, and the United States? Once again, the problem we’re having right now is in supply chain. It’s hard to say that we would have been able to build to the exponential need even if an open design were ready from the start, because similar supply chain issues would have presented themselves.
However, I do think that the “software nightmare” associated with new designs is something for which open source is extremely well suited. A highly scrutinized, well maintained, open source software stack would be a powerful asset when looking for solutions to a shortage such as this one.
The hardware side of things is a bit more difficult to envision as an open source project, simply because contributors to the project would need to be able to replicate the hardware — a problem faced by all open hardware projects. It’s not impossible, but maintaining a bill of materials that is widely available is extremely challenging. However, with mechanical drawings, CAD files, thorough specifications, and a superb testing regime, the task in a time of crisis becomes engineering around the specific gaps in your supply chain, rather than perfectly replicating the design.
Maintaining a design is also crucial. Will equipment manufactured today be possible to manufacture ten years from now without major redesign? Will the features still be relevant for our needs in ten years? Open source is powerful, but abandon-ware is less so. The open source community has many success stories about projects living long lives as maintainers pass the torch from one to the next.
Simply put, open source is people, if the community remains, so does the project.
Need for Ventilators Beyond COVID-19
One of the major problems we face is that ventilators are a low-volume medical product. There are a whole lot more washing machines out there than ventilators, and washers are cheap. There’s much more frequent need for washing machines, and if they don’t work right the consequence is merely a load of clothes that didn’t get clean. When you produce a lifesaving device to meet rigorous regulatory standards in small volumes the price ends up being very high. Open source projects are not free as in beer — it takes time and resources to build prototypes and have them certified. But once established the designs can be used without payment. If a design can meet the safety standards, the potential for widespread manufacture is an uplifting concept.
I’m saddened to learn that Nigeria has something like 500 ventilators for all of its 200,000,000 in population. Compare that to the United States with about 160,000 for a population of 330,000,000. In a time of crisis like this, we need a well reasoned system of sharing equipment and personnel across borders and oceans. I’ve seen some indication that this is happening with both Oregon and California loaning ventilators to New York, hopefully bridging the gap until those new ventilator supply chains mentioned earlier pay off. I hope this type of sharing will accelerate and be extended to all areas in need.
Once the crisis has passed, life-saving tools published as open designs could be one path toward greater availability. Nigeria’s number of ventilators sounds very low to me considering its population. Could countries in this situation take on their own manufacturing programs to grow their supply using dependable, tested, open source designs? That is a future I’d like see.














I could see a niche for an open source ventilator, longer term project, that would survive well in storage. All features optimized for being mothballed for decades. Maybe it features quick knockdown for ease of storage and shipment, with easily removable elastomeric parts that can be “pickled” in glycerine or something to defer deterioration. Only components with the longest known shelf lives used.
I like that idea in theory. However, the reality is that such a thing just doesn’t happen. Most elastomers break down over time due to things like elevated or even just room temperature or oxygen or leeching or reacting plasticizers. The “PTFE like” ones that tend to resist this will still creep over time and not provide as good of a sealant. Many lubricants don’t hold up or interact with other materials over a long period of time. Corrosion, well, happens. The only question is at what rate? Many common (cheap) medical raw materials that are designed for single use are made from materials like PVC plastic which leech out over time, lowering their plasticity (as well as not being terribly healthy to begin with). Batteries don’t hold a charge indefinitely and storing batteries empty can destroy them. There are only so many raw materials that you can use as elastomers and they all have their own plusses and minuses for different applications. You can’t just pick “the best one” since it doesn’t really work like that.
Just look at some of the current ventilator units that are coming out of longer term storage (and even those require someone to do periodic maintenance work on them). They don’t all work and that happens at a rate that’s much higher than you might expect. Unless you specifically design it with some essential components that have to be sealed and partially reassembled before use (and even then some materials just don’t have the same physical properties after a decade or more), I struggle to believe that you could store every part and take things out of storage and immediately put them to use with 100% efficiency.
Could it be done? Probably. But you would really, really need to scrutinize the long term viability of everything that goes into them, depending on the timeframe you needed these to be stored for and how frequent the periodic maintenance would be performed. You can’t just seal up 50,000 or 100,000 and then unearth them a decade later, plug them in and they all suddenly spring to life and work perfectly. If they are identical designs, it’s even possible that none of them work.
I guess if you could design everything from scratch with the specific design intent to be stored for long periods of time with several build in redundancies, seal everything from oxygen and moisture, keep it at low temperatures and at low humidity and have a method to quickly put things together and certify them to be in working status, ensure every moving part was compatible with a method to redistribute internal lubrication when turned back on, have spare parts both stored but also easy to manufacture as well as design everything internal to be sealed and able to resist liquids, you could probably achieve this goal and even do so at a reasonable price point if enough were made. I am not an expert in the nuances of ventilators but I also don’t know of any that are designed for this except maybe some very niche ones to be used for something like in a space environment or something equivalent?
Speaking of the requirements for items on a space vessel, this reminds me a bit of how you design a computer for a space vessel that needs to have built in redundancies. Both from a hardware but also the software side. For example, the space shuttle had a Primary Avionics Software System (PASS) computers and a completely independent Backup Flight System (BFS) computer. Running on multiple, independent hardware units. Really interesting and the software side of this is just as important as the hardware side. https://www.nap.edu/read/2222/chapter/5
The downside to all of this right now is that you would need to develop this whole thing now since it will take you many months to develop, test, manufacture and store this and will not do much to handle the current crisis. If you need a quick solution that sort of works better than nothing, this isn’t the solution you are looking for. But it’s still something that feels like a really good project to pursue (as opposed to trying to quickly retool automobile plants).
The downside would be if it is not open source. Miring something like this in patent law and profit seeking and closed contracts is antithetical to an open sourced, highly efficacious and life saving end result. Nobody has actually built something like this and even the “open sourcing” being done here by one company isn’t really open source. These are life savings devices. What prevents the open source community or someone else who makes it open source from stepping up and developing something truly viable and well engineered?
As much as I appreciate the innovation attempts from MIT and other endeavors and as better as they are compared to no solutions, you really need to check all of the requirement boxes for this to be a safe and viable solution. Many of the ones shown in this article are just not professional enough in scale and scope and manufacturability and COTS product sourcing and overall design to be close to a viable solution at this time. Maybe it comes down to the fact that you are basically asking for something that is fundamentally challenging with a very widespread number of required items and functionality that all have to be well addressed while remaining well designed, easily maintained, inexpensive (to a degree) and will not be a short term endeavor. Throw in unprofessionals and don’t pay anyone anything and I guess it’s not terribly surprising why such an endeavor hasn’t exactly happened yet. Is there a way to solve that problem somehow? Clearly there are enough professional and experienced people out there in the world as a whole to be able to come together and achieve this.
open source medical equipment is a good thing. is a ventilator really worth 30,000 dollars??? its a box with a microcontroller and actuators/valves/sensors. a 25 dollar raspberry Pi board has all the processing power needed……knobs (potentiometers) or touch screen -~ around 20 to 50 dollars cost……….. take the “OOOH – AAAAAh” emotions away … its just a very simple “robot” ….. every hospital bed should have a ventilator!
Open source medical equipment or ventilators specifically here could certainly be a good thing. Such a device could most certainly be built for far less than $30,000 each in quantity, once you amortize development costs and even after factoring in using quality materials and high end engineering, testing and certifications.
However, it still needs to be more than simply a single relatively low priced commercial duty microcontroller and a few commercial quality actuators, valves and sensors. This is literally life saving equipment that needs to function in the real world without failures or downtime or even 1:1 oversight either. Which means it needs redundancies and inherent robust functionality, similar to what you would see and expect for space flight or other aeronautical development environments. I like the Raspberry Pi but it’s not exactly what I would consider suitably engineered for life saving purposes by itself or as it is currently produced.
Could you design or modify it or something similar and then build the resulting product from the ground up to be a real time OS based set of core functionality, combined with visualization hardware for the operator? Sure. But you would need to actually put the development time in to do so and then produce them in quantity and presumably have funding from somewhere to be able to do so. They would not be $20 to $50 each though at that point. But they also wouldn’t be $2000 to $5000 either. Plus the rest of the setup would all also need to be designed, evaluated, tested and revised as well.
FFS, the cost is about extremely high design, validation, certification and testing costs for a low volume product. It’s not mainly about the cost of the BOM.
Still don’t understand why people need this explained to them.
And a raspi has plenty of processing power, but runs a whole OS and so lacks reliability for a safety critical real-time task.
I’m surprised this needs to be explained, but in open source the design and validation is volunteer work, and in this case the certification can be donated too.
So it is only about the BOM and direct manufacturing costs.
It’s never going to work acceptably until we nationalize healthcare. We will always have excessive prices (no matter what the justification is; I think it’s breathtakingly naive for anyone to claim there’s no disaster profiteering going on, or excessive price gouging happening in private healthcare in general). Not everything in the universe has to be a viable, profitable, market-based business. That’s pure ideology. We’d never have gotten humans on the moon using privatized nonsense. Healthcare is fundamentally a public utility, should be treated as such, and if this current catastrophe and the great depression we’re about to dive into isn’t enough to convince people of that then I don’t know what else can possibly be said. Go with god, I guess. We’re all gonna suffer for the sake of this terrible Reaganized bullshit. Just look at how the US is performing with corona compared to the entire rest of the world. It would be embarrassing if it weren’t so god-damn horrific.
For perishable components, store the manufacturing tooling (injection molds, jigs etc.) with the devices, along with copies of all the specifications, chemical formulae, software source code etc so that if repairs need to be made on the stored devices they can be.
That assumes you have access to a room-sized 50 ton molding machine to put the mold in….
Not really, with good design most pieces could be stainless steel or similar durable stuff, sealed bearings, etc and then just a few pieces, like seals and tubing would need to be manufactured or kept on hand. Those parts could be optimized for quick manufacture, using say standard size tubes and clamps with seals or gaskets cut from sheets of material with a vinyl cutter or water jet. Connectors might be 3D printed and so on. We know how to store electronics and machinery for long periods, the Army does it all the time.
I recently spent 4 hours making 80 surgical masks out of cleverly shaped pieces of Goretex interfacing for sports apparel. No sewing or skilled labor, just tab in slot on a piece of cleverly shaped “fabric”.
Good design solves problems as long as the problem is well defined.
Great article.
The software problem was referenced in comments to one of the previous Hackaday articles on ventilators.
It referred to “Therac-25 and Patient Injury”
One such site it http://users.csc.calpoly.edu/~jdalbey/SWE/Papers/THERAC25.html
Ah, I know that story well. Adam wrote a great article about it: https://hackaday.com/2015/10/26/killed-by-a-machine-the-therac-25/
What we really need is an overhaul of the FDA legislation, and new laws that open up the free market to allow more companies to manufacture medical devices, especially in an emergency, with out fear of reprisals. I am the proud owner of a small engineering company that specializes in electro optic sensors. We have the earned luxury of multiple in house heavy duty CNC machines, as well as an excellent staff of world class engineers. In response to current events (and because it was a fun challenge) we designed and built an excellent ventilator. This is a complete unit with truly innovative multi sensor biological feedback. We did it in 1 weeks time. HOWEVER, our legal team laid out in great detail the absolute horror that would unfold if we were to go in to production of these devices. There is a fist full of FDA and medical approvals that must be bought / gotten, and then there is the USPTO. In order to get the unit to the market, with the current legal system, think 1.5 years and 2×10^6 dollars. You know you have problems when you are measuring dollars in scientific notation.
We need legal reform, especially in the medical community. Do you want to be ready for the next big medical / bio crisis? If so, remove the chains of crony-capitalism (a term I hate by the way, because it is not capitalist ) and let the free market / FOSS communities provide for you.
The approvals are there for a reason. We don’t want patients dying in the middle of the night because their ventilator stopped working, or because it delivered them an electrical shock.
Guys like you don’t get it. The machine has to work right all the time, not 90% of the time, all the time. And it it fails it should be able to do so in a manner that does not kill the patient.
That means lots of testing, safety interlocks up the wazoo, every line of code documented, etc. Something that is costly and alien to the FOSS basement dwellers.
The FDA’s role is to make sure the machine works as advertised so that when a hospital buys one, it knows that it is safe and works and BTW that there is a company that stands behind the machine and supports it 7/365.
Would I trust a device that didn’t go through FDA cert? No. Nor should anyone else.
I do get it. I don’t want to be killed by faulty equipment any more than anyone else. Do I want to be hooked up to a ventilator made our of 3D-printed PLA ball bearings and Arduino code written by a 6 th graders?? Hell no.
My point is that it is NOT as impossible a task as people have mad it out to be to build a device that is not going to kill people by means of crappy design and build practices.
what I am saying is that many “ordinary” companies build devices, that if they fail, could lead to loss of life situations all of the time. They do this very safely and effectively, without –excess– government oversight. Every light switch in your house can kill you / start a fire. Every inch of Romex in your house is a potential fire starter / killer. The hydraulic hoses in your cars braking system are “life support” that you use thousands of times per week. All of these devices are made with out FDA approval.
I am NOT saying that the entire legal system should be thrown away. What I am saying is that the current system needs to be overhauled / updated / fixed. As it is now, it stifles very capable companies from solving very solvable problems.
There are definitely regulations for light switches and automotive hydraulics, or basically anything else that can affect people’s life or health. Obviously, the rules for a light switch are simpler than for a complex medical device with many possible fault modes.
There’s no simple solution. When somebody wants to design a device, they want simple rules. When someone loses a life, they want more complex rules.
Should learn from 737 MAX that self regulation is insufficient when profit over safety in said industry. Regulatory capture is a thing too for companies that are too big to fail.
So, it was in Boeings best interest to build a plane that kills people? Their profits went up because of this? And the “crack team” of FAA /NTSB engineers found the problem before the first crash?
You are conflating your (Nobel) desire to live in a completely safe world with the reality that there is risk in everything, including engineering. Should we throw away every cancer drug because they inadvertently kill 5% of the people they try and cure?
“So, it was in Boeings best interest to build a plane that kills people?” is the QA responsible then???? or is this bs to employ more QA admins per engineer/technician? where does the buck stop??? with the QA admin that did not follow due diligence???? theres a lot of blood on QA hands!!!!
Part of what’s made the current legal mess is people filing lawsuits, and winning, over medical problems that were 100% unforeseeable, unpredictable, and when they occurred, uncontrollable. Sue for “malpractice” when someone dies during a heart operation because their heart muscle was too diseased to hold sutures – which there’s no way to tell until the operation is underway.
Have you noticed every drug advertisement says do not take this drug if you’re allergic to it? How can a physician tell if you’ll have a bad reaction until you take it? It’s not possible. If the reaction is bad enough it may kill the person. Several years ago an allergy medication was taken off the market because it *may* have contributed to the deaths of a few people. What discontinuing the drug did was cause a lot of harm to people with asthma and allergies because common asthma drugs and allergies don’t get along. IIRC that’s why that different allergy drug was developed.
So rather than come up with a test to see if people would have a bad reaction to the drug, it was discontinued to avoid lawsuits.
Malpractice lawsuits should be restricted to provable negligence or incompetence of doctors and other medical practitioners, and for devices and medicines put into production with known but undisclosed problems or defects. Or for cases where something was used for a purpose for which it wasn’t intended, like Teflon implants for TMJ pain.
Teflon is considered to be biocompatible, but only if it stays together. When a Teflon implant was developed for pain caused by misalignment of the TMJ (jaw to skull) joints, it got approval in part based on the known biocompatible nature of the material. But what wasn’t considered was what happens to Teflon when put under the compression and rotating shear forces the TMJ experiences. Several people ended up with major damage to their jaws and skulls due to the body’s reaction to microscopic particles of Teflon. But what really made that wide open for a lawsuit is the implant manufacturer and doctors continued to push the implants after the early failures came to be known. The testing was inadequate, and that’s in part on the FDA for granting approval for a novel use of Teflon without considering the very different use conditions.
But the FDA never pays when it screws up. (Just like the EPA never pays when it screws up and causes pollution.)
teflon (brand name for ptfe) is tricky. it can leach out weird gasses at moderate-high temp depending on the brand of ptfe.
In my experience all the different plastics (especially thermoset) are tricky and not to be trusted at face value and need use testing.
Agreed!
But aren’t we talking about last-resort measures for use in a time of crisis? I’d rather take my chances on sketchy equipment than simply be allowed to die because there isn’t enough to go around. Wouldn’t you chose that too?
We don’t want an innovative device. We want devices built to existing specs that medics know how to use, and can be trusted.
Med Device Engineer here. I lead a team of ppl that deliver class III devices. Yeah, it’s hard but it’s because killing 1 person in the us is a big thing. That is the core. The USFDA’s core tenet is safety first.
Gonna be a little snarky here – because I don’t see anyone being really open about the difficulty –
If you feel so confident you can deliver without killing people (ventilators are something I have heard many horror stories about actually) then sell in Europe. You can go to market in 6 mos or less with way less money. They have way relaxed regulations compared to the US – they determined many years ago that more startup entrants and new technology outweighed safety.
But I will be real with you, if you are the lead and you have never done this before you probably will kill someone. (It’s inevitable to some degree even if you have experience). I think you are overestimating what you can do without experience in the area. The guys I know that have built ventilators have described horror stories where leaving a software button invisible from a test mode cancelled operation, stuff like that. A minor crash that’s a corner case kills someone, all kinds off stuff. It’s a totally different way of looking at product from consumer stuff. You can reboot a chromecast. there’s nothing like that for the poor nurse you are going to leave on an island because you decided to ship a minor defect anyway.
Lastly – the ventilator is not a true gamechanger where if you build a better mousetrap and DISRUPT it will help. A ventilator *might* save 10-20% of people, but most people on ventilators are not gonna make it anyway. I leave the real success to the scientists working on a vaccine. On an actuarial basis speaking as someone in the industry, it sucks but the best thing 95% of us that make hw/sw can do is not go outside and let the scientists get their money to make a real silver bullet, a drug.
So, your position is “everything is fine, leave the current systems of regulation alone” ?
Sure, the regulations probably need an overhaul. Rewriting them now during an emergency? Bad idea. That deregulation you want so you can sell your untested ventilator? Big pharma would love it.
Deregulation of medical devices is a scary idea.
I would only argue that the law should have provisions for times of emergency when there are not enough approved supplies available. I would also agree that during the emergency is not the time to write those laws. Changes will not be ready in time for this emergency anyway. Right now there are probably more urgent things for lawmakers to do. It’s good to think and talk about them though because it should happen as soon as it is over in preparation for next time.
Also, none of that matters unless someone does come up with a design that will save more lives than it kills and can be produced in times of need without competing for resources with the production of better, FDA approved devices. Isn’t that the step developers are at now? Just trying to determine what if anything the open source community can do?
Yes, I admit it… You’ve got me all figured out… I want to sell untested, unsafe, poorly designed equipment with my name on it and harm as many people as possible in The middle of a national crisis. Seriously?
Except we have real life examples of money being chosen over safety – design wise Boeing they should have build new airplane not redesign 737 but they where afraid to loose customers (pilots get certified to fly certain types of airplanes, when new model flies the same as old they don’t need for new certification and cost of certification is big thousands of $ for each pilot with big fleet bill gets bigggggg) and out of pure malicious greed Prudue Pharma(and other drug companies) and their push towards selling more OxyContin.
I would challenge you as to what you would think is reasonable. The USFDA just really requires design control and FTA/FMEA. It’s not that hard. It’s just a pain to be thoughtful and thorough and write it all down. I hazard that your world class engineering team can do it, they just don’t wanna take the time.
You make a good point. Where there is an emergency, and the alternative is getting no ventilator at all, and certain death, the regulations should be waived or relaxed. In the UK we have a fast track approval for such devices as long as they conform to a basic specification. Even if you device works for 95% of patients that’s quite a few lives saved.
The JMA Wireless folks also (apparently) produced a AMBU/BVM based design:
https://jmawireless.com/prevail-ny/
http://www.jmawireless.com/share/PREVAILNY-R1.zip
Unclear to me if its a truly bespoke or based on one of the other similar designs.
I worked on the JMA design and prior to that the MIT group’s MIT E-vent. The design is rapidly scalable and has now pressure sensing making it a true “Assist Control” device. It has plateau pressure recording with settable alarms for the PIP. The design is a more robust mechanical actuation and it is made from COTS parts and aluminum. This design was also tested in ARDS animal models and provided safe ventilation post injury. Paper to follow. Cost is roughly $500 per unit in the U.S.
The level of Quality Assurance required for a software product is usually based on the consequences of a fault/failure in the product. For me, the levels were basically:
Non-critical – Minor annoyance.
Business Critical – A business partially or completely stops running.
Safety-Significant – A hazardous operation runs without correct monitoring or oversight.
Safety-Critical – One or more of your employees gets injured or killed.
Life-Critical – One or more members of the general public gets injured or killed.
Our overall software QA planning specified that each application got rated, and an appropriate QA plan had to be developed for anything non-critical. The key word here is “appropriate”. The cost, complexity, and level of effort required for hardware/software design and manufacturing processes goes up exponentially with each step of the ladder.
Another key word here is “required”. In highly regulated industries like medical devices, deliberately ignoring or bypassing QA is not an option. In fact, it is a go-to-jail prosecutable Federal crime. In my particular case, I became very familiar with DOE Order 414.1
These requirements exist for a reason. Unfortunately, the reason is usually based on a body count among the general population. Like the old joke says: “building codes are written in fire and blood”.
When you step into one of the regulated industries, you are stepping into another world. Like military planning, the time to prepare is before the battle starts,
I am building a ventilator from off the shelf industrial automation supplies. They do have to be oxygen cleaned, but I’ve found several places that can do that for me. The design is modeled after the all-pneumatic ventilators from Penlon and Pneupac:https://drive.google.com/open?id=1YYCX9BjlgQJXe7zbC5GEnGrYUrKuFWQk
Medical devices have lots of FDA requirements for good reasons: if something unexpected happens, people die. We have an extremely low tolerance for faults resulting in death. Vioxx and fenfluramine both worked, but they also both damaged and killed a small proportion of the people who used them, so they were taken off the market and their respective manufacturers were both successfully sued for truly enormous amounts of money. The problem of people assessing culpability for an action, but not assessing culpability for lack of an action, may be a logical fallacy. Nobody sues a company for not making ventilators when people who need them are dying, but they’ll surely sue a company making ventilators that don’t work right.
There’s also a side issue that once a system of regulation is firmly established, companies who have to live up to those regulations have an incentive to make the regulation system more complicated and expensive to hamper new competition. This is related to regulatory capture.
There’s an obvious argument to be made that if someone’s going to die anyway, why not try out this DIY thing? Where I live there are Good Samaritan laws, whose purpose is that if you make a good-faith effort to save someone’s life and end up injuring them in the process, you generally can’t be held liable for those injuries. However, at least where I live, those laws only apply to people who have no medical training. If you’re a doctor or an EMT, you can be held liable if your life-saving attempt ends up paralyzing a person, because you’re supposed to know better. Making DIY emergency medical equipment is fundamentally different than encountering an injured person: it implies knowledge and planning. I don’t think there’s anything like a legal framework for providing limited liability to people making DIY medical stuff even if the recipients are going to die without it. Maybe there should be, but I don’t think US culture at least is ever going to accommodate that.
https://www.corovent.cz/, full open source. Next week starting production of 500 pcs.
It´s not fully opensource. The license is bizarre and one has to really hurry to use it. https://ventilation.fbmi.cvut.cz/corovent/
Not even slightly open source! The “temporary open” license expires 31 May 2020, but even then the conditions that apply are highly restrictive.
When the crisis ends (Which will probably not be by may 31) low grade ventilators will be worthless.
Hospitals will keep the high grade ventilators (Which can be used to cover all low grade and high grade use cases) and ditch the low grade ones, which are the ones where they are sharing the design under restrictive licensing.
I don’t know what these companies think is going to happen when we have 100,000 ventilators in an area that has a normal need for 100-200. Absolute best case scenario these things end up in a strategic reserve, but since the places that make them under an “open source” license are unlikely to offer maintenance contracts, they’ll be spare parts in a matter of 5-10 years.
Seems like mandating that the specs be followed, and mandatory inspection following the crisis with a mandatory maintenance contract for any that pass inspection and are kept in service or in a reserve would be a better way to proceed.
It is still open source, just not free (as in freedom) or OSI approved. Do not mix those terms please.
This is not a hobby project. It is an emergency project into which the university and the industry here invested quite a lot of money and knowledge. They even submitted the devices to proper regulatory authorities to make sure this can be officially used in local hospitals.
The real answer is to not denigrate from political pulpits Universities and Biological Research institutions because they give uncomfortable predictions about the biological realities of climate change, and fully fund them so they can also work on solving the biological reality of pandemic problems, and having prepared the tool chains for finding cheap effective vaccines quickly. Ventilators are a practical response, and to be admired, but not a solution for a country like Nigeria and only a stop gap measure for us, whereas a vaccine is. When any Joe on the street, loaded up with invisible viruses is far more dangerous than a terrorist with a suicide vest on, then the whole western paradigm that the community’s economic and technological wealth, divorced from biological reality, is more important than the total communitiy’s health is turned on it head. To get our response right, we must first get our priorities right. Do we want to be arguing about which bandaid is best today, or really prepare for life on earth over the next thousand years?
I submitted this earlier but Medtronic has open sourced their ventilator… source code, cad files, schematics.
https://www.medtronic.com/us-en/e/open-files.html
They may have *published* a design, but it’s definitely not open source.
It seems to me that this “COVID 19 Warning: This Ventilator was created for use only in response to the COVID 19 pandemic. It is provided AS IS. ” makes any sort of license other than the one they offer impossible. What they have done is provide an emergency situation with a quick fix, by publishing their own existing closed-source design, for which they paid for the R&D, and the testing to gain FDA approval, etc. For a true fully open-source design they would have find some way for their own FDA approval to cover what you made! From what I know of how the US system works, apart from them then being liable for your screw-ups, I don’t see how that could be done. Don’t look a gift horse in the mouth!
You need to get the oxygen in.
it would be interesting to hear the thoughts of those that subscribe to the theory of Natural Selection.
Uh, I’m pretty sure there is more than one way to do that. A ventilator is EXTREMELY invasive.
Regulation is the cause of the slow developement in much of medicine.
Regulation isn’t a bad thing per se, it’s needed, but it does have short commings.
As well as a barrier to entry which limits competition allowing prices to rise, it can be costly to meet the regulations which in of itself means prices need to rise.
As with all critical equiptment prices need to be high to cover indemnity costs.
I wonder if the fear and trauma of be intubated makes people ‘give up’.
Hi Mike, thanks for the great analysis. Our group recently released a unique open source ventilator design, which could be an interesting alternative for the current approach of ambu bag squeezing and PVC pipe glueing. It’s a pressure controlled, fully 3D printable ventilator that consists of 2 pieces of plastic.The design is based on an old military ventilator. For anyone interested in the project here’s a link (also if you want to learn more about how it works check out the Coanda effect and fluidic amplifier ventilators):
https://github.com/MSwoboda/yvent
Good. And finally, you Hacks are getting on it.
It is also worth noting that a lot of places in the world would see an affordable but bare bones ventilation device as a huge improvement. One of the guys on the Harvard ventilator project, which predates covid, was inspired to work on it after he spent hours squeezing an ambobag, working shifts with the family and friends of a sick child in a Pakistani hospital with no better technology. The child died, probably, at least in part, because the bag had no controls and less feedback than even the most rudimentary design here.
Anyone who has followed 3D printer evolution will understand that open source designs drive down prices. Many parts of the world cannot afford what Western folks consider the basics of medical care, things like blood pressure cuffs and otoscopes. A ventilator that could be afforded and maintained by third world hospitals would in a few years save many more lives than will be lost to covid-19
It looks like the priming bulb to my boats fuel supply. Glad to know I am set :)
Hi everyone.
Brief idea for _non-tested_ breathing-assist type device.
Plastic?/metal? 44 Gallon drum.
Different sized plastic bottles (1500mL ==> 250mL) mounted internally to walls of drum.
Probably need seals on inside of bottles, between bottledrum.
External compressor (probably high volume) controlled by solenoid?, dumps air into drum, compressing multiple plastic bottles simultaneously, forcing air out each bottle neck.
Would need one-way air intake valve in-line on outlet of each plastic bottle, for replacement air intake.
Solenoid for drum air-exhaust.
(+++)
– Large number of users per unit
– Low cost to produce (labour excluded – biggest time factor would be removing?/replacing? drum lid? in order to install plastic bottles)
– Basic parts (drum/bottle) would be available generally world-wide – compressor/control gear, not so much
– Variable size for different age patients – possibly able to scale smaller for infant use???
– One life helped/saved = worth it. I hope this helps someone, somewhere, sometime…
(—)
– Big problems if unit fails…
– Lifespan of plastic bottles undergoing continuous expansion/contraction – going off-of anecdotal evidence (a.k.a my personal plastic water bottle, and how long they last), hopefully these units would last a day, maybe more…how many people have thrown out their plastic bottle, just from it looking terrible from use (plastic fatigue?), and how many have thrown it out because the bottle physically failed..?
– Re-inflation of bottles would happen by ??? – ? vacuum compressor (utilise air-compressor intake???)?
Far more lives will be saved with an open source solution to manufacturing affordable insulin. Only half of the people in need will be expected to be able to get the insulin they need by the year 2030. https://www.cnn.com/2018/11/21/health/global-insulin-shortage-diabetes-intl/index.html The current process using genetically modified ecoli has a manufacturing cost of $43000 per kg (about $1 for a box of 5 pens, which retails for $424 in the US) https://gh.bmj.com/content/3/5/e000850
One of the problems now facing hospitals in the UK is oxygen plumbing though the building, loss of the standard 4psi at the ports at the bedside with so many ventilators hanging off the pipework in multiple wards. Designs must conserve medical oxygen and not be wasteful.
We just did it.
eAR project is working on forced ventilation mode.
eAR was designed to meet specs of MIT Project E-VENT and MHRA (UK) – Rapidly Manufactured Ventilator System
Characteristics:
– Tidal Volume SET: 200-800ml range, calibrated at 400ml
– BPM SET: 8 to 32
– I/E Ratio: 1:1, 1:2, 1:3 and 1:4
– Plateau Hold Time: 150, 250, 500 and 1s
– Overpressure cutoff on 1 cm H2O resolution. Overpressure observed is less than 1 cm H2O.
– Mandatory External Watchdog Timer
– Works on simplest mechanics available:
– car window lift motor (right or left type)
– car fuel tank float sensor
– laser cut MDF parts, optionally use acrylic or metal sheet parts
Updated info on Github: https://github.com/RespiradorHacker/Projeto-EAR-Celso
With a BOM around 50 USD (for ambu automation)
This fellow is a doctor in an NYC ICU & thinks we’d be better off just putting people on oxygen than using ventlators
https://youtu.be/k9GYTc53r2o
The Montreal General Hospital Foundation and the Research Institute of the McGill University Health Centre launched a Ventilator Challenge on March 19.
According to them: “The judges will evaluate which of the submissions are most likely to be of lower cost, higher quality, greater reliability, greater ease of production, greater ease of use, greater modularity/repairability, uncompromised safety, and aesthetics”
They received entries from 1029 teams from 94 countries. Nine (9) semi-finalists have been selected and the entries are now being subjected to an intensive series of tests by medical professionals in Montreal. The top three designs will be announced on May 6.
https://www.mghfoundation.com/en/news/code-life-ventilator-challenge-testing-phase/