What’s the coolest thing a person can build with LEGO? Well it’s gotta be an air conditioner, right? Technically, [Manoj Nathwani] built a LEGO-fied swamp cooler, but it’s been too hot in London to argue the difference.
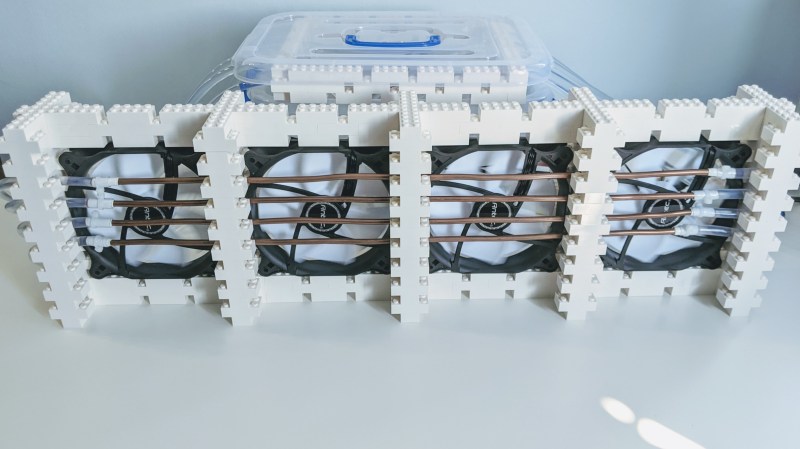
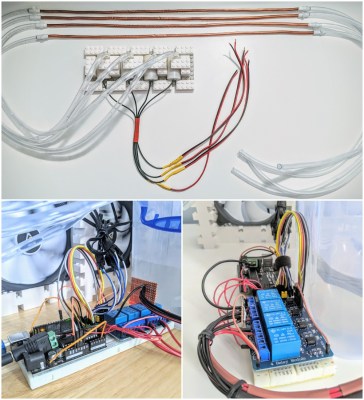 This thoroughly modular design uses an Arduino Uno and a relay module to drive four submersible pumps. The pumps are mounted on a LEGO base and sunk into a tub filled with water and ice packs. In the middle of the water lines are lengths of copper tubing that carry it past four 120mm PC case fans to spread the coolness. It works well, it’s quiet, and it was cheap to build. Doesn’t get much cooler than that.
This thoroughly modular design uses an Arduino Uno and a relay module to drive four submersible pumps. The pumps are mounted on a LEGO base and sunk into a tub filled with water and ice packs. In the middle of the water lines are lengths of copper tubing that carry it past four 120mm PC case fans to spread the coolness. It works well, it’s quiet, and it was cheap to build. Doesn’t get much cooler than that.
[Manoj] had to do a bit of clever coupling to keep the tubing transitions from leaking. All it took was a bit of electrical tape to add girth to the copper tubes, and a zip tie used as a little hose clamp.
We think the LEGO part of this build looks great. [Manoj] says they did it by the seat of their pants, and lucked out because the copper and plastic tubing both route perfectly through the space of a 1x1x1 brick.
DIY cooling can take many forms. It really just depends what kind of building blocks you have at your disposal. We’ve even seen an A/C built from a water heater.















It’s not an A/C, but it’s not a swamp cooler either since it doesn’t use evaporative cooling! It’s just using ice water to cool down the air via the copper pipes.
Maybe it could be called an “air-water heat exchanger?”
(and this is absolutely not a criticism of the project – if it works, it works!)
Also, I’m anticipating a comment from someone who argues it really *is* an a/c, albeit one with a remote compressor and a weird manual intermediary stage, since presumably the ice comes from a compressor-driven fridge.
More of a heat exchanger. This design heats up your kitchen, and then cools down the place where you run it. If both of those things are in the same, enclosed space, the net effect is higher heat.
This is a pretty dumb idea for nearly any use case, as as noted, it certainly isn’t a swamp cooler.
“a system for controlling the humidity, ventilation, and temperature in a building or vehicle, typically to maintain a cool atmosphere in warm conditions.” so yea.. it’s an air conditioner.
Correct. Most of the other names are refinements/subcategories. In this case, I think the most accurate would be cold-deck cooler or chilled-water cooler (there are other names that amount to the same: chilled water from a remote source- mechanical chiller, geothermal, wherever is used as the to absorb heat from air inthe conditioned space). In large plants, such as a large office building, there will be a hot water and a cold water circ system feeding the hot and cold decks (heat exchangers) in each air handler, and water-flow rates through the decks are adjusted to control temperature. Typically, one or more air handlers are in each space, though there may be larger handlers for overall building make-up air and/or climate control. (this is an incomplete description, but gets the idea across. I haven’t used this professionally in better than 20 years, so will go no further)
Forgot: In hte US, cooling is measured in tons, which refers to the heat required to melt or freeze n tons (US conventional, about 90% of a metric tonne) of ice per day. One ton of cooling is 12000BTU, which is two small to mid-size window rattlers at about 500W each, or one of the largest window units in the US. Came back when mechanical refrigeration was primarily used in ice plants, before most homes and businesses had electric refrigeration or air chilling.
This puts that measure in clear perspective.
Would this cooler be measured in ounces? ;)
A required component of ac is humidity reduction.
This is a heat exchanger, and if the refrigerator is in the same flat, this will raise the average temperature. Some 70% of ac energy goes into removing moisture. In short, I don’t see here a complete thermodynamic theory of cooling a living space.
The thermodynamic concept at play might be that, even if the average temperature throughout the domicile rises, the temperature gradient that forms from such a system still could result in a lower temperature at the area occupied. This area could reasonably assumed to be relatively distal from the kitchen or fridge room, so there’s an argument to be made that this apparatus could still reasonably be expected to improve comfort for the bean.
This likely won’t cool the living space as a whole but would provide a fan-driven stream of cooler-than-ambient air into a small area, which may cool things in that area slightly better than a fan-driven stream of ambient temperature air would.
More surface area for the copper tubing part would probably help, though. Could probably just solder lengths of copper tubing to the current tubing to provide perpendicular radiation surfaces.
Since the temperature of the water in the pipes is relatively cold, bellow the dew point of the room, water vapor is going to condense on the pipes… So technicaly it is going to reduce the amount of water in the air.
This summer, I worked in my caravan. I tried to lower the temperature using ice, and did some maths … 1 kg of ice would lower the temperature by 2°C in ideal condition …
Hypothesis: 1 kg of ice at -15°C
Temperature inside the caravan: 35°C
Volume of my caravan: ~ 3 m3 (it’s an Eriba Puck, the smallest caravan I know)
=> ( (1 x -15 x 400 ) + (3000 x 35) ) / (3000 + 1) = 32.99 °C
(400 is a “constant” a frigorist adviced me for the calorific power of the water against air)
So I am not sure of the real benefit to cool a whole room.
Are there some frigorist or expert that would confirm or infirm my calculus ?
Ok. I’m not good at this. Its 25++ ago I done that last time…
To bring 1 kg of water from -15° C to 0° C it needs 30.900 J. To melt the ice it needs 333.000 J. To go from 0° C to 30° C it needs 125.430 J. So in total it needs about 489.330 J.
3 m^3 air is about 3,612 kg. To bring this air down from 30° C to 29° C it needs -3.630,06 J.
Your body (as far as I know your body…) needs 2000 kcal a day which is 8373,68 kJ. So every hour you produce about 350.000 J.
Draw your own conclusions.
Thats some heavy air you’ve got there…
It’s 333,000J (333kJ) to melt 1kg of ice. Working it all out, it ends up at a similar energy density as running a normal A/C from lithium batteries. Water is far cheaper than batteries (not to mention far safer to work with) so it ends up making a great option for storing solar energy for air conditioning.
new automotive heat exchangers go for less than 20 € on ebay. transfer should be way better than some copper pipes.
383039897076 371122684903 293732495403 383613998166 164054727762
Or a 240 to 480mm rad from a pc water cooling loop.
Some large buildings don’t use a compressed/expanding gas cycle system directly for air cooling. They use such a system to freeze tanks of water solid during times when the building isn’t occupied. A lot of pipe runs through the ice tanks and up to heat exchangers in the air ductwork. The pipes are filled with a liquid that has a lower freezing temperature than water.
To make a small scale system for small area cooling it would be most efficient to use a heater core from a vehicle and make an insulated container that will fit into your freezer with its lid off. Fit the container with a lot of small copper tubing, ideally several loops in parallel to have better flow. Use small dry break quick connects like are used on those cold therapy devices that are often a modified small cooler into which ice and water are put, then the chilled water runs through a pad with tubes.
The idea is to use the freezer to remove heat from the water, then use the ex-heater core to remove heat from the air and put it into the ice to make it phase change to water. The insulated container keeps heat from outside of it from prematurely melting the water, and also keeps the heat put into it through the air blown through the ex-heater core from getting back into the room.
But since most home freezers pump the heat from inside themselves into the room + the heat from the inefficiencies of the system, this would still just be temporarily shifting heat from the small space its air blows into.
Air conditioners do more than just cool the air, that can happen though many functions or processes. Air conditioners specifically cool and dehumidify the air though the refrigeration process by transferring energy from one space to another via mechanical means
This might save your arse on a hot day out in the shop, but its just time shifting the refrigeration process though storage and distribution. Its like saying “I watched a show live by freezing it on DVD and replaying it later”
Thus its a stored energy heat exchanger, its nothing magical, have one at my bench for decades now, runs off a 98 cent ice pack and a fan
Anyone know what the purpose of the Arduino is? It’s not entirely clear from the blog post.
to boot up Raspberry Pee
Hmmm, some tubular 1×1 LEGO pieces could be drilled through and used for some of the tubing!
B^)
I really appreciate you for publishing this blog here about this lego air conditioner is cooler than yours; it’s really a helpful and very useful for us. This is really appreciated that you have presented this data over here, I love all the information shared. Great article!Come across Comfortgroup.co.nz and hope you can visit this too to get more information.
Since the temperature of the water in the pipes is relatively cold, bellow the dew point of the room, water vapor is going to condense on the pipes… So technicaly it is going to reduce the amount of water in the air.
“An efficient HVAC system keeps you cool in summer and warm in winter.”
“An efficient HVAC system ensures year-round comfort and indoor air quality.”