Belgian, Italian, and Australian researchers are proposing that by stacking semiconductor sheets, they should be able to observe superconducting behavior at what is known as “kitchen temperature” or temperatures you could get in a household freezer. That’s not quite as good as room temperature, but it isn’t bad, either. The paper is a bit technical but there is a very accessible write-up at Sci-Tech Daily that gives a good explanation.
Superconductors show no loss but currently require very cold temperatures outside of a few special cases. The new material exploits the idea that an electron and a hole in a semiconducting material will have a strong attraction to each other and will form a pair known as an exciton. Excitons move in a superfluid state which should exhibit superconductivity regardless of the temperature. However, the attraction is so strong that in conventional materials, the excitons only exist for the briefest blip of time before they cancel each other out.
The new material has layers and manages the holes in one layer and the electrons in the other. The pair will move together but are not in danger of canceling each other out as they stay on their layer. As you might guess, that means one layer has p doping and the next layer will have n doping.
The team claims that their predictions are they will see superconducting currents in these 3D stacks at temperatures up to -3 °C which is only about 26 °F. Cold, but not crazy cold. Liquid nitrogen, for example, is nearly -200 °C and dry ice is about -80 °C. Compared to those, -3 °C is almost balmy.
(Editor’s note: it’s -3 °C outside our house right now.)
The researchers also think they will be able to raise the temperature with more work. As of now, the paper is just a proposal, although the researchers say that building the stacks doesn’t rely on any unusual technology. It will be interesting to see if anyone can implement superconducting materials using this method.
While building this lattice of alternating semiconductor seems hard, we’ve seen hackers do harder things. It is possible to make superconducting ceramic that works at relatively high temperatures. We have seen one room-temperature superconductor, but there’s a big catch to it that makes it pretty impractical for real use.

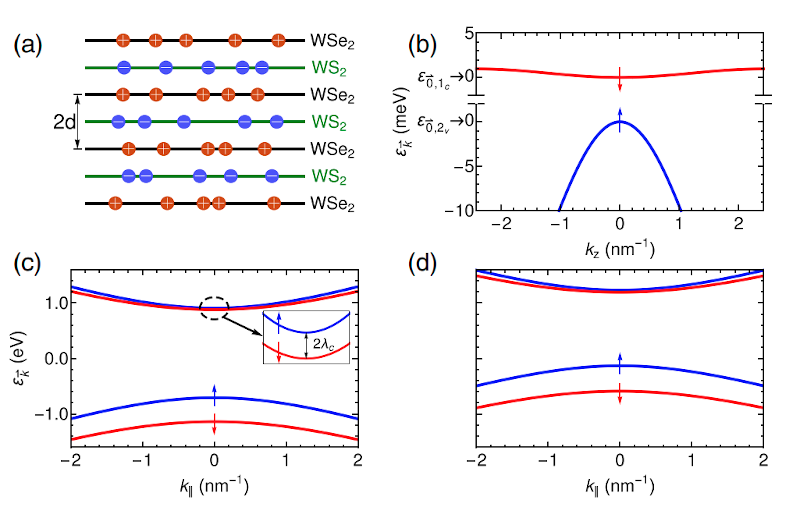














-3 °C for a superconductor is fantastic.
Yup, that’s easily doable in the “kitchen”, yer olde salt and ice bath that is used for ice cream making will hold it below that.
Yea MRI machines run with regular R134a instead of liquid Helium!
Sadly no to the MRI using R134a instead of liquid Helium
I learned this below from z24-a6n. The exciton can transport energy without transporting net electric charge. No net movement of electric charge means that there will be no magnetic field generated, which is a key requirement for a MRI.
What pressure is this achieved at?
We’ve all been clamouring for this for decades
=P
Came here to find out what kind of diamond anvil you need to stick this thing into, the paper is paywalled and it doesn’t say in the summary. A superconductor that requires incredible pressures seems overall less practical than one that requires incredibly low temperatures.
That’s not entirely true. You could achieve whatever pressures you need by encapsulation within a substance with mismatched thermal expansion. Then when it is brought to room temperature, there could be incredible stress applied to it by the capsule shirking more than the superconductor. Less practical if it requires pressures that ordinary materials cannot sustain, but otherwise doable.
What keeps the capsule from fracturing under such ludicurous internal stresses?
The diamond anvil consists of a large metal frame, which compresses a wedge-shaped piece of diamond, which tapers down to a point that presses on another diamond of the same shape. If you had the opposite situation with a bit of expanding material inside a diamond freely suspended by nothing, the diamond would simply pop and shatter.
A material with high tensile strength and very little elasticity, rather than a ‘hard’ material like diamond. Think of a cement block – you can support a car on it, but the moment you try to BEND it, it crumbles.
>high tensile strength and very little elasticity
Contradicting requirements. Materials that withstand tension tend to be springy, and materials which aren’t springy tend to be stuff like ceramics which cannot take tension.
>Think of a cement block
Cement is actually pretty damn elastic. Its Young’s modulus is about 30 compared to steel which is 220. It’s even bendier than magnesium. It’s just not very apparent because it cracks so easily under circumstances where the bending would become visible. We build it so big and with such large cross-sections that it simply won’t sag noticeably.
A Prince Ruppert’s drop springs to minds.
Huge internal stress contained by way of topology.
If you need diamond anvil pressures, then sure, it wouldn’t work. If you could get away with less pressure using different superconducting materials, you could achieve fairly high pressures using silicone, ceramics, or even some metals. This already happens when building semiconductors. Usually features are introduced to avoid the build up of stresses from mismatched thermal expansion, but sometimes this is utilized for various reason like thermal contact resistance and MEMS type applications.
No mention of pressure, don’t bother looking anywhere other than Arxiv
https://arxiv.org/abs/1911.01123
As annoying as it was that none of my instituitional access got past that paywall.
> the paper is paywalled
sci-hub.se is your friend
Just calculations, and with no external pressure folded into their calculations it’s presumed to be at room temperature.
Also, with heterostructures/thin films I don’t think applying pressure is very common for practical reasons of not degrading the surface of a 2D (or stacked 2D) material? At least with thin films, you can grow your film on a substrate with a different sized unit cell and achieve the same effect as applying pressure anyway… But I’m not an expert by any means!
Free Version of the paper from Arxiv: https://arxiv.org/pdf/1911.01123.pdf
Now rotate the sheets:
https://hackaday.com/2019/12/27/magic-angle-twisted-bilayer-graphene-yes-thats-the-scientific-name/
If this actually works, is it restricted to DC only ? Since you want the holes on the Tungsten diselenide (WSe2) layers and the electrons on the Tungsten disulfide (WS2) layers ?
I wonder how long the savings, of not having a 8-15% distribution losses, would take to pay for changing all the power generation and distribution from AC to DC, installing kitchen temperature super conductors everywhere.
High voltage DC (HVDC) distribution lines are a real thing and superconducting ones are very desirable. There have been prototype projects with “high temperature” (i.e., liquid nitrogen-cooled) superconductors but this, if it pans out, would be a game changer.
Now while I think the paper is proposing a possible work-around for a prior-theorized restriction on superconducting temperature it’s not a recipe for how to make such materials and the authors didn’t actually try to create one. As such, the paper essentially says “the calculations say building materials with these characteristics should superconduct at higher temperatures; implementation is left as an exercise for the reader.”
True, but Tungsten diselenide is currently being investigated for possible applications in solar cells. And Tungsten disulfide is being investigated by TSMC (Taiwan Semiconductor Manufacturing Company) for possible uses as the channel material in field effect transistors using CVD (chemical vapor deposition). So creating a superconducting laminate of those does have a very good starting point. It is something that the right lab could create initial prototypes almost immediately.
Proposes … and … should. No ‘physical’ testing results. Seems to me, one should have ran some tests before publishing. So take with a grain of salt….
Here is to ‘hoping’, but we’ve been hoping for ‘fusion’ reactors too which still remains theoretical ( here in Earth ) ;) . Be nice to be able to string some Super Conducting wire everywhere some day as needed (or as battery storage devices) ….
Since when are fusion reactors only theoretical?
has one ever made even a single watt hour of electricity?
In the first step, they create heat. Electricity is a possible outcome of it.
And it does create heat, even though in the current state you habe to invest more energy to get it going than we can get out of it.
But it IS a fusion reaction!
Generally speaking in physics, people tend to specialize in either theory or experiment, meaning that the process works something like the following:
– Theorists do some calculations or simulations based on previous experimental and theoretical work and make predictions. This work and its results are communicated to the scientific community by publising papers.
– Experimentalists perform experiments based on previous theoretical and experimental work to test theories (and/or to satisfy curiosity). The results of these experiments are communicated to the scientific community by publishing papers.
– The process is repeated until either the funding or interest in the topic runs out.
The paper referenced in this post is the result of the first bullet point above. The fact that it contains predictions based on calculations and not tested by experiment is perfectly normal and reasonable. The problem comes when pop-science websites aren’t careful with their headlines or the details of their articles (or sometimes when they are but their readers aren’t).
It’s very important to note that the effects described in the paper are not superconductivity in the normal sense of the word, but rather superfluidity of and exciton condensate. This is quite important, especially from the technological point of view that (I suppose) most Hack a Day readers are interested in.
Notably, a superconducting system contains bound states of two electrons (Cooper pairs), so each pair carries a charge of 2e, where e is the charge of an electron. This means that a flow of the superfluid of Cooper pairs carries charge, i.e. a supercurrent of Cooper pairs carries electrical current.
In contrast, the system described here contains bound states of an electron and a hole (called an exciton), so each pair carries zero charge. This means that while the excitons can form a superfluid condensate at low (or perhaps not-so-low) temperatures, a supercurrent of excitons will not carry any electrical current.
This is not to say that an exciton condensate is not interesting, it certainly is, and it may even be technologically useful, but it is not useful for the same things that we use superconductors for (i.e. strong electromagnets). Moreover, it’s quite misleading (and perhaps actually incorrect, depending on how pedantic you want to be) to describe the proposed heterostructure system as a superconductor.
Hm…
A battery is a source of eletric energy. It has an electron donator (connected by the minus pole) and an electron acceptor (connected by the plus pole). The flow from donator to acceptor transfers energy (normally done by two wires: one pushing electrons, one pulling). The system described here transfers two parts (the electron donator: here one electron itself and an electron acceptor: here a so called hole) over one “wire” / material in two electrically seperated layers (at least it sounds the way that electron and hole cannot combine between layers). So basically all you need is a way to split the connections to the two layers at the ends to make the electrons flow through your load before they can combine with the hole.
To me that sounds like a (theoretically) valid way of transporting energy.
Reread your comment…
My definition of a superconductor is as follows: A material that allows charge to flow without restriction (resistens). And since excitons are compost of an electron (has a charge) and a hole (pseudoparticle that has an equivalent but opposite charge of an electron), a material that can transfer these without restriction, is by that definition a superconductor (independent of the state electron and hole are e.g. bound into an exciton or not). Even if it lacks properties other known supercondutors have (like the way they interact with the magnetic field).
So if this material is actually usefull depends on whether electron and hole are truelly bound (in the exciton state) and therefor useless or if they can be combined / separated at the ends.
(In reference to “strong electromagnets”: Actually a condutcor that doesn’t create a (macro scale) magnetic field is an improvement for energy transfer by wire.)
Actually, by your own definition, its not a superconductor since a bound state of an e- and h+ should be treated as a single quasiparticle which is electrically neutral and therefore no charge is flowing.
You wouldn’t say that superfluid helium carries charge without loss, even though it carries 2p+ and 2e- per helium atom…
At the back of my mind was that if there were strong magnetic fields, that would produce a shearing force, which could delaminate the structure. Having no magnetic field at all, sounds useful for laminate structure.
I expect that strong magnetic fields would destroy the exciton condensate, similar to the effect in superconductors, although for different microscopic reasons. I doubt it would do much to the structure of the material.
That said, I haven’t done the calculations, so I could be quite wrong.
I meant like this:
https://www.youtube.com/watch?v=43AeuDvWc0k
But that would only happen if there is a net movement of electric charge. In this case with excitons transporting energy without transporting net electric charge, there is no magnetic field generated. And no magnetic field means that there is nothing creating a force inside the laminate structure.
Reading what you have said make me think that I was not clear enough and you picked me up as saying a strong external magnetic field. And if they are a problem, it could be reduced by encasing the superfluidity of an exciton condensate inside a mu-metal casing.
I see what you mean now, I definitely interpreted your previous comment as referring to an external magnetic field.