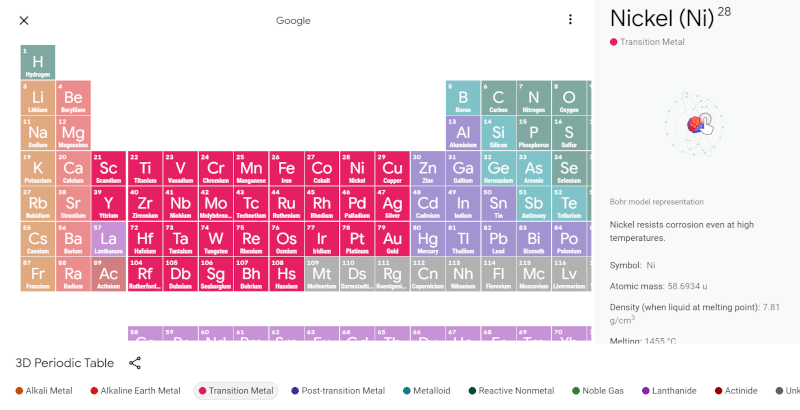
One of the nice things about the Internet is that you don’t need huge reference books anymore. You really don’t need big wall charts, either. A case in point: what science classroom didn’t have a periodic table of the elements? Now you can just look up an interactive one from Google. They say it is 3D and we suppose that’s the animations of the Bohr model for each atom. You can debate if it is a good idea to show people Bohr models or not, but it is what most of us learned, after all.
While the website is probably aimed more at students, it is a handy way to look up element properties and it is visually attractive, too. You probably remember, the columns are no accident in a periodic table, so the actual format doesn’t vary from one instance of it to another. However, we liked the col coding and the information panel that appears when you click on an element.
Not that we haven’t seen interactive online tables before. There’s Ptable, for example, or one from a Royal Society, no less. If you want to go commercial, there’s always Fisher — a well-known name around the lab. Their table is pretty simple from a technical point of view, but they have longer writeups about each element.
Granted, we don’t reach for a periodic table every day. But we do need some of that data sometimes. If you need a refresher on what to do with it, talk to [Will Sweatman]. If you prefer to make everything a game, try periodic table Battleship. Meanwhile, for extra credit, try figuring out what other elements are missing from [Tom Lehrer’s] song in the video below without looking at the tables.















The internet is an amazing library if you know how to search it. It seems my friends have issues with
a) picking the right keywords
b) filtering garbage, finding the relevant information
Let me know if anyone has a “how to Google it” guide. I don’t remember how I learned, it’s all intuitive now
I often know what keywords will separate the information i want from what the search engine thinks is relevant. Doesn’t help at all if Google believes it’s model of me knows better. If the advertising model says “information about x is more relevant” then all the keywords in the world won’t convince it otherwise.
Sometimes logging out and being anonymous is the best option.
You mean you don’t use “remove cookies after closing browser” option? 😯
You can exclude results from a Google search that contain particular words or phrases by using a dash before a word, so for example searching for “donuts” (without the quotes) would return results about any type of donut, but “donut -glazed” (again without the quotes) would exclude any results that contain the word glazed.
You meant hyphen. It matters.
It’s really a minus sign. It matters.
And then Amazon finds the exact item you’re searching for and puts it on page 3 so you dig through all the other items you might buy.
You got that right.
Sometimes changing the order of keywords can change the results
The worst is when someone has named a product after the item or topic you want or used it in a popular meme. Like if there is a tool in metalworking called a rickroll.
In my experience its actually best to NOT search for keywords, or at least not at the start, (depending on subject) thats how you get a lot of useless results, instead i often search the (English) sentence i would use to ask others for more information.
That way you usually end up getting better results (as google will literally go “hey this sentence appeared here too” wich would then link you to some discussion somewhere that’s exactly about what you want to know)
If this tactic doesn’t work (as is the case for some specific subjects) i start stripping out words from the sentence so it becomes more and more of a keywords search, but i always go with the sentence first
Quite often my biggest headache is Pinterest. Why does it even exist?
keyword keyword keyword -pinterest.com
I know exactly what you mean Maave, and I have solved the Google search problem by NOT using Google; I use Duckduckgo, which, by the way, does not spy on you.
DDG may not spy on me, but the past few searches for datasheet resulted in ads from chip foundries, not datasheets.
Nor does duckduck censor sites like google does.
Ironically (at least for me), just a few days ago I fulfilled a decades old promise I had made to myself to order a copy of the CRC Handbook of Chemistry and Physics from the same year I graduated college (1990, 71st edition).
Why do I want it? I don’t know. When was the last time I looked in one? I don’t know. What will I do with it? Other than flip through it and put it on a shelf, I don’t know. On the other hand, thanks to the same internet it was really easy to find one, and it was cheap.
At least I can look forward to my death, when some relative will have to touch it to either dump it in a box of donations or throw it away. Ahhh, Memories…
https://hackaday.com/tag/crc-handbook/
🤣 I believe I FINALLY donated my 1985 edition of the same book to our local Goodwill recently!
omg thats so kawai
67 mango diddy blud ohio rizzler
Bohr models are fine for chemistry which is all about sharing electrons. Really. That’s all of it.
No they are NOT. Chemistry cares about the shapes of the orbitals. Electrons can’t be just anywhere and that affects how molecules can go together. The Bohr model is incredibly confusing and misleading for chemistry.
Instead of wasting time and effort making misleading little balls go around in nonexistent circles that represent absolutely nothing, Google could have put some thought into visually representing something sort of like a realistic electron cloud, but NOOOOOO… some graphic designer wanted to make little balls go around and around.
And then they added 3D rotation, which gives the false impression that you learn something interesting by viewing the totally meaningless picture from a different angle.
I don’t think that’s even the real Bohr model. I don’t remember a bunch of coplanar circular orbits corresponding to shells.
Nice!
I’m not a gun at chemistry, but I do know the electron orbiting model was gone before the internet came.
I agree, all we have here is some doode with too much time creating a flashy looking graphic that impressed the execs.
You are thinking PChem which is applied quantum mechanics and is a physics course for chemists. It stresses the interaction of light and matter. The kind of chemistry where you need a periodic table just counts electrons. Is it covalent? Ionic? How strong? Electron counts and electronegativity. You can get picky and count hydrogen ions in solutions as protons but it is still about counting electrons. The maps of expectation values that form the clouds are studied briefly in any High School chem course and never used again. Electron energy level diagrams look like the Bohr orbitals so it is only natural don’t you think?
For anything other than high-school chemistry you are going to need to know the real shape of the orbitals, not just for quantum chemistry but for pretty much anything. For instance, the reactivity and properties of many transition-metal complexes is determined by the interaction of d-orbitals on the metal with orbitals on the ligands. The orbital shape is key to this.
Yes, and all off molecular biology is about the shapes and the fields. But the chart shown here is basically grade school or middle school material. And that is what I was trying to describe. It is definitely lacking for a high school course don’t you think? Full disclosure I have a secondary teacher’s certificate for AP physics and math with a chemistry endorsement of the “we can’t find anyone else” type and surprise, they couldn’t! So I have been through various high school texts and state exams in excruciating detail.
If you are taking high school chemistry the you need a real table with electronegativity and a bunch more info. Maybe Google will expand this into a useful tool.
I read this whole thing just for the Tom Lehrer clip. :-D
You have until 2024-12-31 before this site is shutdown to enjoy all his lyrics – https://tomlehrersongs.com/
He is 93 now, very cool of him to move all his works (excluding any existing music that he does not own the rights) into the public domain now.
Well the lyrics and sheet music, which if you check on the internet archive has been growing over time.
Oh, this hasn’t been done before lol.
I still have one in my wallet and pull it out at least once a week.
Now periodic table is a Google thing, too?
Periodic videos is a great place to learn things and have fun whith theese colomns and rows :
http://www.periodicvideos.com/index.htm
professor Martyn Polyakoff is a fantastic guide ;0).
Don’t see unobtanium on there.
They couldn’t find any to put on it!
It’s still a hot mess. Lanthanum and actinium are f block elements, but this chart botches that in fine tradition, cramming them in under scandium in the d block. But then they also went and threw in a gap to the right of scandium, which implies that the f block fits over there. Why not just put that gap to the left of scandium, put La and Ac down in f where they belong?
Table layout differ, c.f. how Google table follows the Royal Society of Chemistry table since neither lanthanum and actinium have f shell orbitals and so do not inhabit the f-block. They are however chemically lanthanide respectively actinide [well, duh]. Wikipedia on the f-block: “The two 14-member rows of the f-block elements are sometimes confused with the lanthanides and the actinides, which are names for sets of elements based on chemical properties more so than electron configurations. The lanthanides are the 15 elements running from lanthanum (La) to lutetium (Lu); the actinides are the 15 elements running from actinium (Ac) to lawrencium (Lr).”
don’t all elements have f-shell orbitals? (maybe empty ones).
I had the same reaction about the Bohr model – it’s more an art project than science (in which case you want orbitals who oddly makes for more artsy illustrations as well).
Case in point, the H description botches the cosmology and have H as “75 %” of all matter instead of 15 % if you count the dark matter as a newtonian and 4 % if you count the Comar mass of the stress-energy tensor as in relativity. (Which is a useful approximation for a slowly expanding space as ours.)
I like Atomistry since it includes synthesis techniques from a number of old public domain chemistry books: http://atomistry.com/
Maybe some of the data on that site is out of date, but some of the knowledge is impossible to find in modern books.
The old public domain chemistry books are a good resource for learning the basics how the elements can be separated and how to make basic chemicals. They are a better source for knowledge than modern resources unless perhaps you have institutional access to databases of synthesis protocols, which seemingly aren’t available to individuals.
That’s a good one. Also has nice collection of the element in various PDB protein structures.
Another good online periodic table is:
https://www.webelements.com/
Thanks [Experienced Experimenter] and [eckythump] for the links. I like the clean (fast!) layout of both those pages. They look a bit Web 1.0 from back when dialup was slow and people went online looking for information instead of distraction.
I should share a link too. This one generates nice printable PDF periodic tables. You can select how much info gets printed to make the table more relevant to your field (or just to reduce clutter), then you can put your school or company logo on the top to personalise it: https://norris.org.au/expet/ptab/
I don’t see what’s so impressive about it. My chem book in high school had the same info in it and I didn’t even need internet connectivity to use it.
Or am I missing something?